KEY CONCEPTS
- The minerals present in metabasites (metamorphosed mafic rocks) vary with metamorphic grade.
- Most metabasites form by metamorphism of high-temperature igneous protoliths such as basalt.
- Low-grade metamorphism of mafic rocks commonly involves high-temperature minerals reequilibrating to lower-temperature conditions.
- Many of the minerals in metabasites have variable and complex compositions and, consequently, metamorphic reaction are continuous and do not plot as lines on phase diagrams.
- A metamorphic facies is a general range of P-T conditions that produces distinctive looking rocks.
- We differentiate facies based on the metamorphic minerals present, on rock color and fabric, and on grain size.
- A facies series is a sequence of different metamorphic zones that increase in grade across a metamorphic terrane. Facies series reflect the settings in which metamorphism occurred.
- Different minerals and mineral assemblages characterize different facies and facies series.
- Ultramafic rocks that originate as part of the oceanic lithosphere, may become serpentinites.
- Serpentinites and other ultramafic rocks may be obducted and become part of alpine peridotites and continental lithosphere.
13.1 The Minerals in Metamorphosed Mafic Rocks
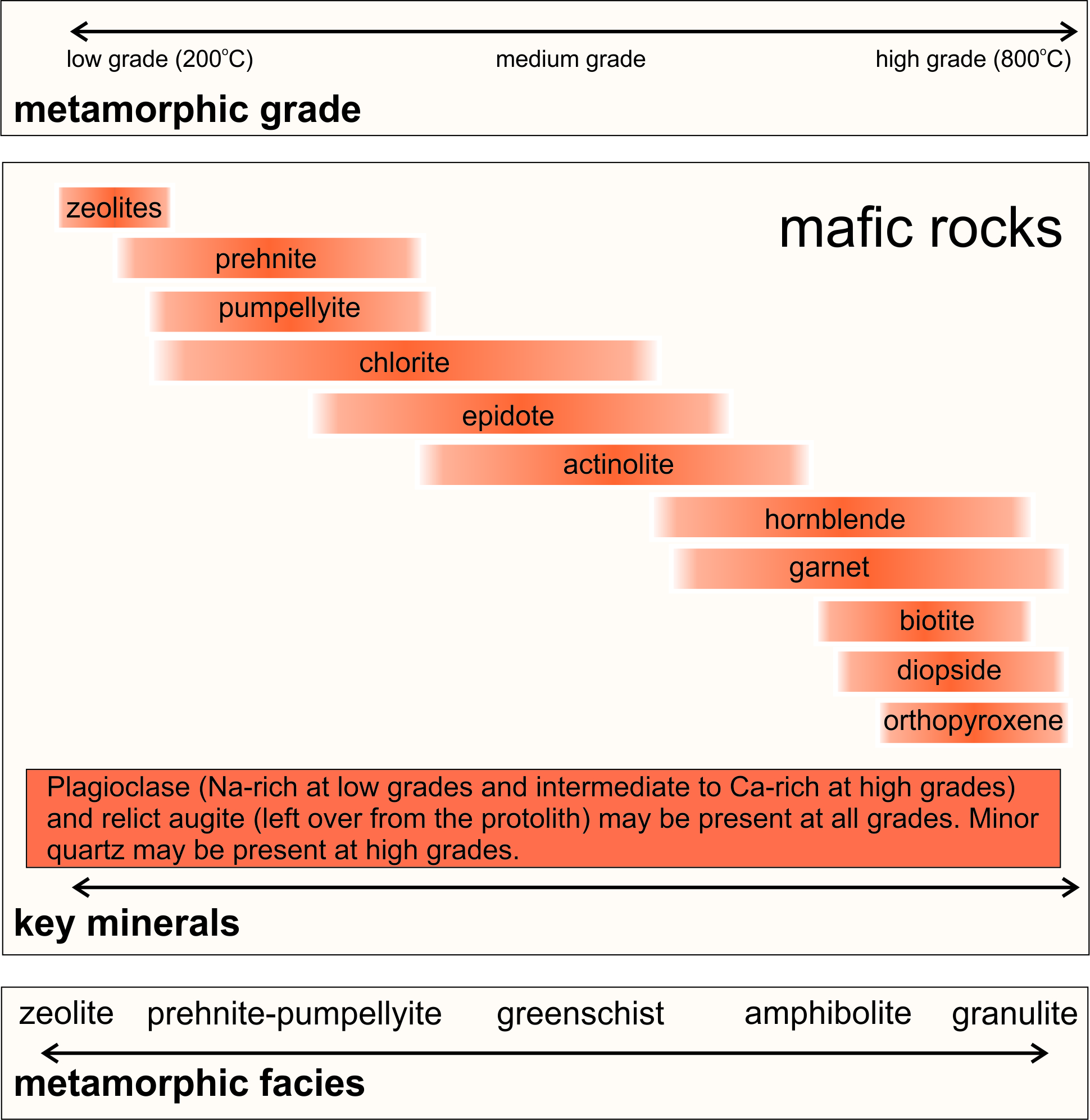
Figure 13.1 shows some common minerals found in metabasites (metamorphosed mafic rocks) at different metamorphic grades. A spectrum of minerals is possible, beginning with zeolites, prehnite, and pumpellyite, at low grade. And, ending with hornblende, garnet, biotite, diopside, or orthopyroxene at high grade. Plagioclase is stable at all grades.
Many of the minerals in Figure 13.1 are only stable over discreet temperature ranges. Thus, the minerals or mineral assemblage in a rock tell us the general metamorphic grade. Lower-grade minerals, including zeolites, prehnite, pumpellyite, chlorite, and epidote are all hydrous. Higher-grade minerals, including garnet, diopside, and orthopyroxene, are not.
Most metabasites form by metamorphism of high-temperature mafic igneous rocks, commonly basalt. Consequently, during low-grade metamorphism, new minerals develop as high-temperature minerals reequilibrate to lower-temperature conditions. This is not true for metasedimentary rocks because, in those rocks, the protolith minerals formed at low temperature.
Table 13.1 lists the compositions of the minerals included in Figure 13.1. One complication with metabasites is that many minerals in mafic rocks have quite variable compositions – and thus variable stability ranges. So, the sizes of the red boxes and their order in Figure 13.1 are approximate.
| Table 13.1 Common Metamorphic Minerals in Mafic Rocks | ||
| grade | minerals | formulas |
low grade high grade |
zeolite prehnite pumpellyite chlorite epidote actinolite hornblende garnet biotite augite orthopyroxene |
variable Ca-Na aluminous silicates Ca2Al2Si3O10(OH)2 Ca2(Mg,Fe)Al2Si3O11(OH)2•H2O (Mg,Fe)5Al(AlSi3O10)(OH)8 Ca2(Fe,Al)Al2Si3O12(OH) Ca2(Fe,Mg)5Si8O22(OH)2 amphibole with complex chemistry (Fe,Mg,Ca)3Al2Si3O12 K(Mg,Fe)3(AlSi3)O10(OH)2 (Ca,Na)(Mg,Fe,Al)(Si,Al)2O6 (Mg,Fe)2Si2O6 |
13.1.1 Variations as Metamorphic Grade Increases
At the lowest grades, zeolite minerals, prehnite, and pumpellyite form during diagenesis and the onset of metamorphism of mafic rocks. Often, these first metamorphic minerals fill vugs or fractures, and the overall rock may still have the appearance of the protolith. At slightly higher grades, chlorite, epidote, and the green amphibole actinolite form as rocks become greenschists. And, commonly, relict (left over from the protolith) plagioclase and augite persist at low- and medium-grades. With more metamorphism, garnet and hornblende develop as the lower-grade minerals disappear, and rocks evolve to become amphibolites. Clinopyroxene (generally augite of variable composition) and orthopyroxene characterize the highest grades of metamorphism. Plagioclase is present in rocks of all grades but is generally Na-rich at low grades and more Ca-rich at higher grades. Minor biotite and, sometimes, quartz may be present, too.
Various petrologists have published phase diagrams with reactions relating the minerals seen in Figure 13.1 and listed in Table 13.1. These diagrams have limited value because mafic-rock metamorphism involves many complicated reactions, because different reactions apply to different composition rocks, and because the most important reactions are continuous reactions that do not plot as lines on phase diagrams. For these reasons, when discussing metamorphism of mafic rocks, we often talk about different kinds of rocks and about general changes in mineralogy instead of about specific reactions. The different kinds of rocks define metamorphic facies.
13.2 Metamorphic Facies
Metamorphosed mafic rocks commonly do not develop the same foliated textures as metapelitic rocks, because mafic rocks do not contain significant amounts of micas or other platy minerals. Some metabasites develop weak foliations due to mineral alignment. Some higher-grade rocks may develop gneissic textures, but many metabasites have no foliations at all. So, for mafic rocks, the idea of matching rock types such as slate, phyllite, and schist, with metamorphic grade does not work well. Yet, metamorphosed mafic rocks do have different appearances when metamorphosed to different degrees.

The varying appearances of metabasites led Pentti Eskola to introduce the idea of metamorphic facies in 1920. A metamorphic facies is a general range of P-T conditions that produces distinctive looking rocks. We differentiate the different facies based on the metamorphic minerals present, on color, on fabric, and on grain size. From low-grade to high-grade, the common metamorphic facies are the zeolite, prehnite-pumpellyite, greenschist, amphibolite, and granulite facies.
Figure 13.2 contains photos of rocks metamorphosed in the greenschist and amphibolite facies. Greenschist facies metabasites always have a green color, primarily because they contain chlorite. White plagioclase and black hornblende dominate amphibolites. Grains of these minerals are generally about the same size, so amphibolite often have an overall a grayish salt-and-pepper appearance. Some amphibolites show poorly developed foliation.
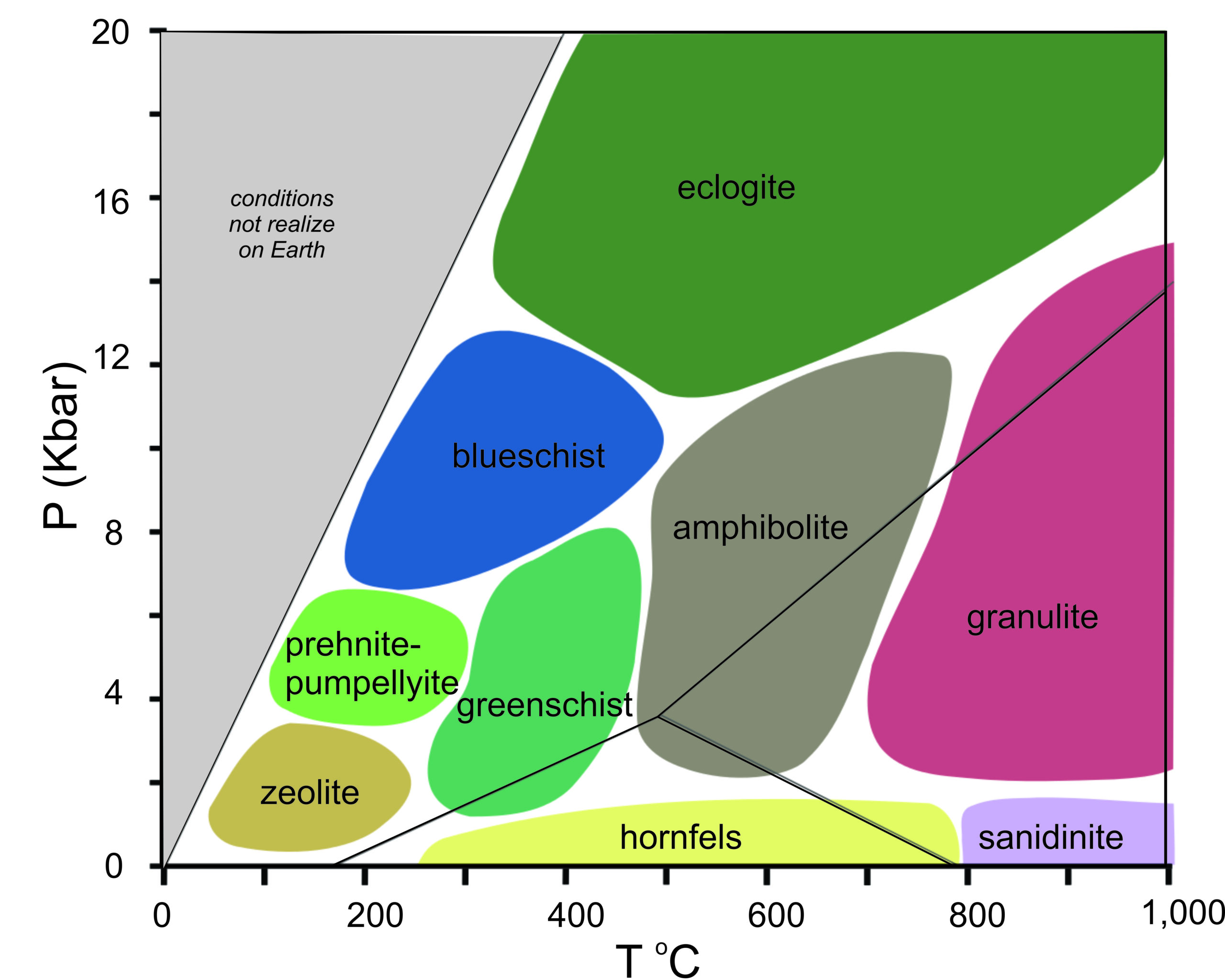
Eskola, and later Francis Turner in the 1960s and 1970s, recognized that some metamorphic rocks form in conditions that are exceptional. So, they extended the idea of metamorphic facies to include all possible P-T conditions at which metamorphic rocks can form (Figure 13.3). In this diagram, conditions in the gray upper-left portion exist nowhere on Earth because it is impossible to get to high pressures (deep in Earth) without substantial temperature increase. In other parts of the diagram, the names of the different facies are the names of different kinds of mafic rocks, or the names of key minerals, that form under different conditions. The boundaries between facies, however, are wide and only approximate.
Figure 13.3 includes the aluminosilicate reactions (black lines separating the stability fields for kyanite, andalusite, and sillimanite) to make it easier to compare the different diagrams in this chapter with diagrams in other chapters. Aluminosilicates, however, never exist in mafic rocks.
Petrologists often use the names of the metamorphic facies when talking about rocks that are not mafic. This can lead to confusion because the names of the facies describe rocks that only form from mafic protoliths. Nonetheless, no matter a rock’s composition, geologists find it convenient to use the same facies names to describe the general range of P-T at which any rock formed. anchor
13.3 Facies Series
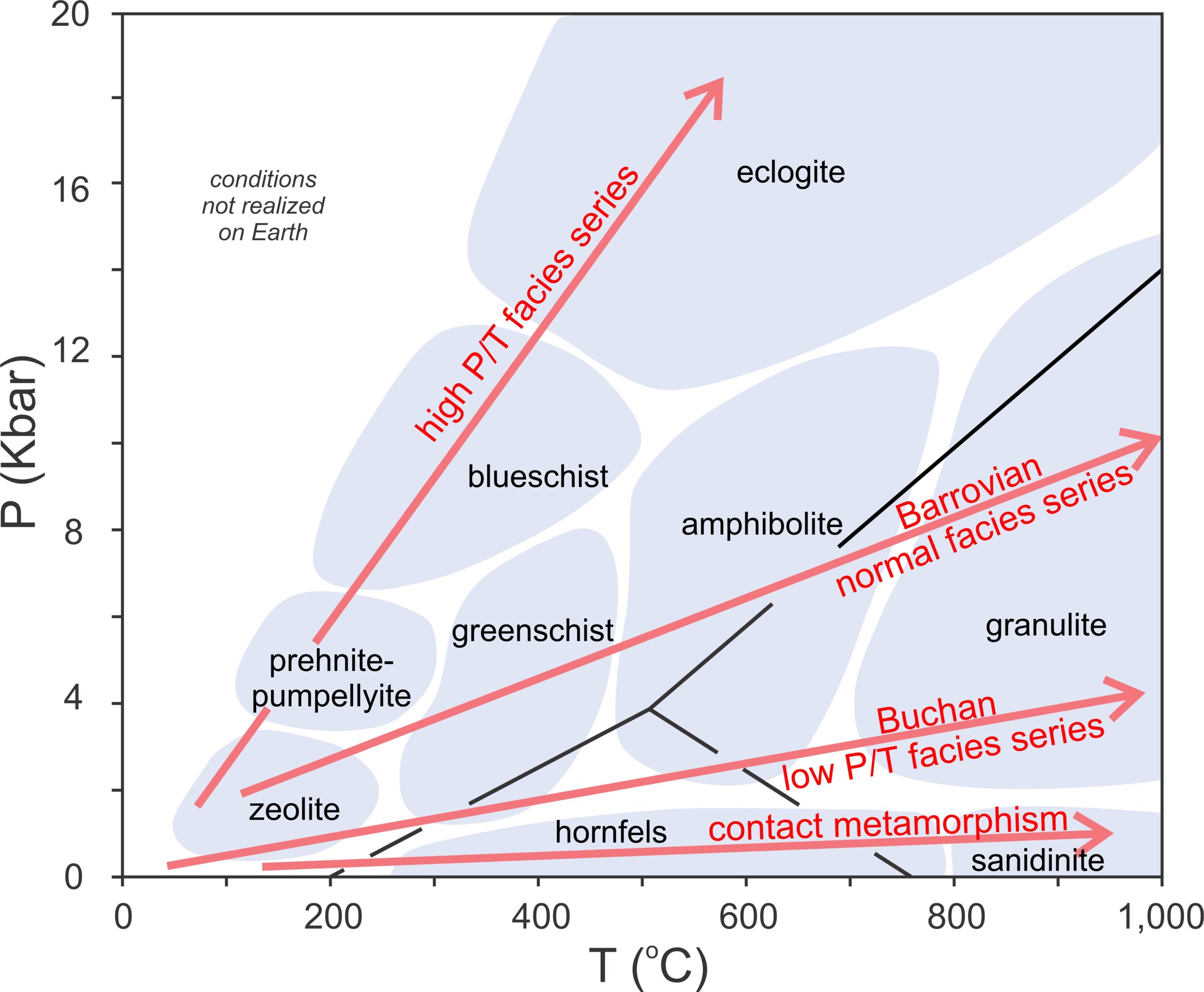
A facies series is a sequence of different metamorphic zones that increase in grade across a metamorphic terrane. Figure 13.4 contains several examples. A Barrovian facies series (named after Scottish geologist George Barrow) follows a normal geothermal gradient created during crustal thickening related to mountain building. Typical Barrovian metamorphism progresses from the zeolite facies to the greenschist, amphibolite, and granulite facies. Rocks in other terranes may follow a Buchan facies series (a lower pressure path of metamorphism named after the Buchan region of Scotland, north of Aberdeen, where it was first described). Buchan metamorphism occurs in places where Earth’s crust is rifting, allowing magmas to move upward while carrying a great deal of heat. It sometimes occurs in other places where high upward heat flow causes metamorphism at elevated temperature without a great increase in pressure. Barrovian and Buchan are the most common facies series, but as seen in Figure 13.4, some terranes reflect a high P/T facies series or a low-pressure contact metamorphism facies series. In either case, the minerals that form will be different from those formed by Barrovian or Buchan metamorphism.
Mafic rocks metamorphosed in a Barrovian or Buchan terrane may appear similar. Unlike metamorphosed pelites, common metabasites, excluding very high-pressure blueschists and eclogites, typically contain no distinctive minerals indicative of higher or lower pressure. But, whatever the facies series, metamorphism of mafic rocks begins with the formation of zeolites. If grade increases sufficiently, they will end up in the high-pressure or low-pressure portion of the granulite facies.
| Chapter 16 Section 16.3.2 contains many photos of metamorphosed mafic and ultramafic rocks in hand specimen and in thin section. If you want to know what they look like, go there. |
13.4 Rocks that Form in Different Facies
13.4.1 The Zeolite and Prehnite-Pumpellyite Facies
Low-grade metabasites may appear as altered basalt (or other altered rocks), and distinguishing low-grade metamorphism from alteration is often impossible. With the onset of significant metamorphism, the first new minerals to form are usually zeolites – there are many different kinds. Mafic rocks generally contain plagioclase, and most zeolites have compositions related to hydrated feldspars.
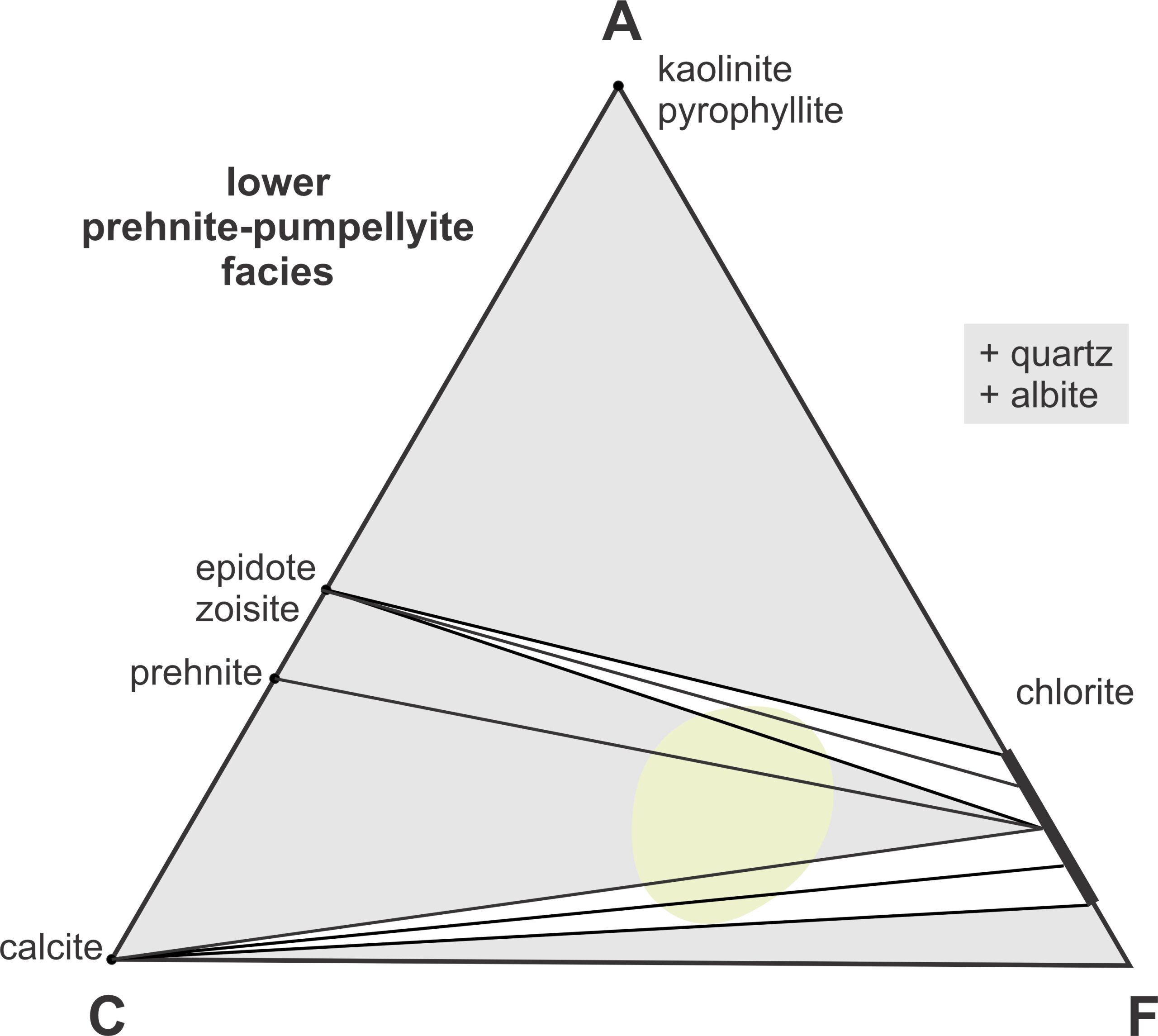
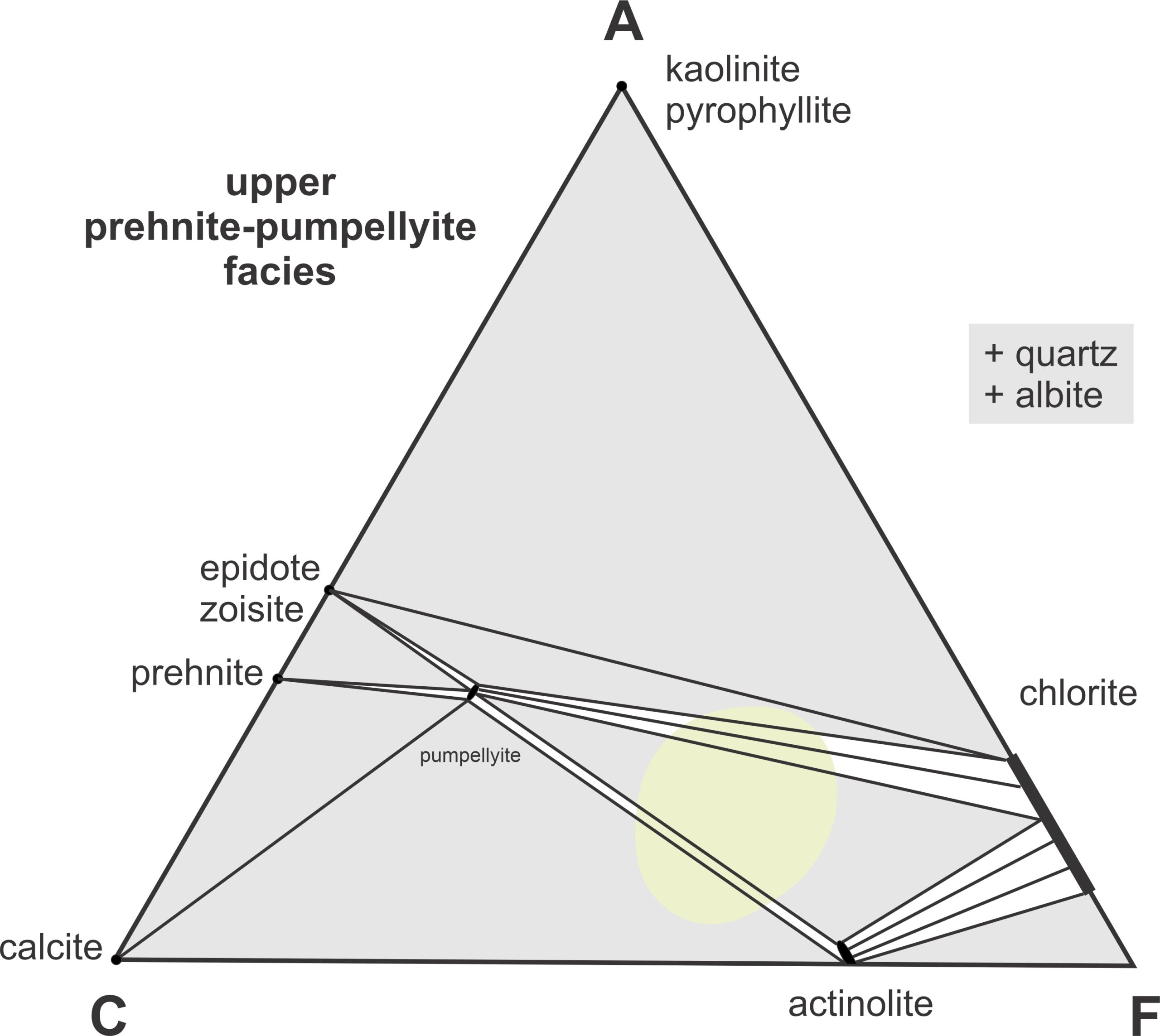
Rocks that form within the zeolite or prehnite-pumpellyite facies generally contain distinctive zeolite minerals, prehnite, or pumpellyite. Figure 13.5 depicts typical mineral assemblages in these two facies. In this and other ACF diagrams in this chapter, 3-phase fields are gray and 2-phase fields are white with tie lines. The light green region depicts the typical compositions of basalt and other mafic rocks.
|
|
|
Diagenesis overlaps with metamorphism, and some zeolites like heulandite, wairakite, or laumontite can form both during diagenesis and metamorphism (Figure 13.5). But some zeolites are exclusively metamorphic minerals. With further heating, prehnite may appear (Figure 13.6). The early forming metamorphic minerals have hydrothermal origins and form mostly in vugs, veins, and other openings in the parent rock. They may, for instance, appear in vesicles in metabasalt.
Zeolites are quite hydrous and unstable at elevated temperatures. When zeolites disappear completely, usually around 200 oC, metamorphism reaches the lower prehnite-pumpellyite facies. Epidote appears and then pumpellyite (Figure 13.6). At temperatures around 250 oC, actinolite forms and the most common assemblages in the upper prehnite-pumpellyite faces are pumpellyite-actinolite-chlorite and pumpellyite-actinolite-calcite (Figure 13.7). In contrast with the lower-temperature minerals, actinolite generally crystallizes within a rock mass instead of in vugs or veins.
The photos below are three examples of low-grade metabasites. Figure 13.8 is a basalt containing vesicles filled with zeolite minerals. Figure 13.9 is another basalt, this one metamorphosed to prehnite-pumpellyite grade. The white minerals are mostly chabazite, a zeolite. And Figure 13.10 is a photo of a metabasite containing radiating crystals of pumpellyite.
13.4.2 The Greenschist Facies

In some geological terranes, metabasites skip the lowest facies, and the first clearly metamorphic minerals are chlorite, epidote, and actinolite. These minerals define the greenschist facies and are responsible for the green color that gives the facies its name. Greenschists may develop schistosity due to alignment of chlorite and actinolite crystals. Figure 13.11 depicts the most common assemblages. Pumpellyite and prehnite are absent from greenschists because temperatures are too high for them to be stable; albite and quartz are generally present. Chloritoid may also be present but is uncommon.
|
|
The figures above are photos of typical greenschist facies rocks. Figure 13.12 is a greenschist outcrop in Japan. The photos in Figures 13.13 and 13.14 are hand specimens of greenschist. Greenschists are often very fine grained and the minerals present must be inferred. The hand specimens in these photos, however, contain coarse chlorite and actinolite. Figure 13.2 shows another example of a greenschist; it has a different texture than the two samples seen here.
13.4.3 The Amphibolite Facies
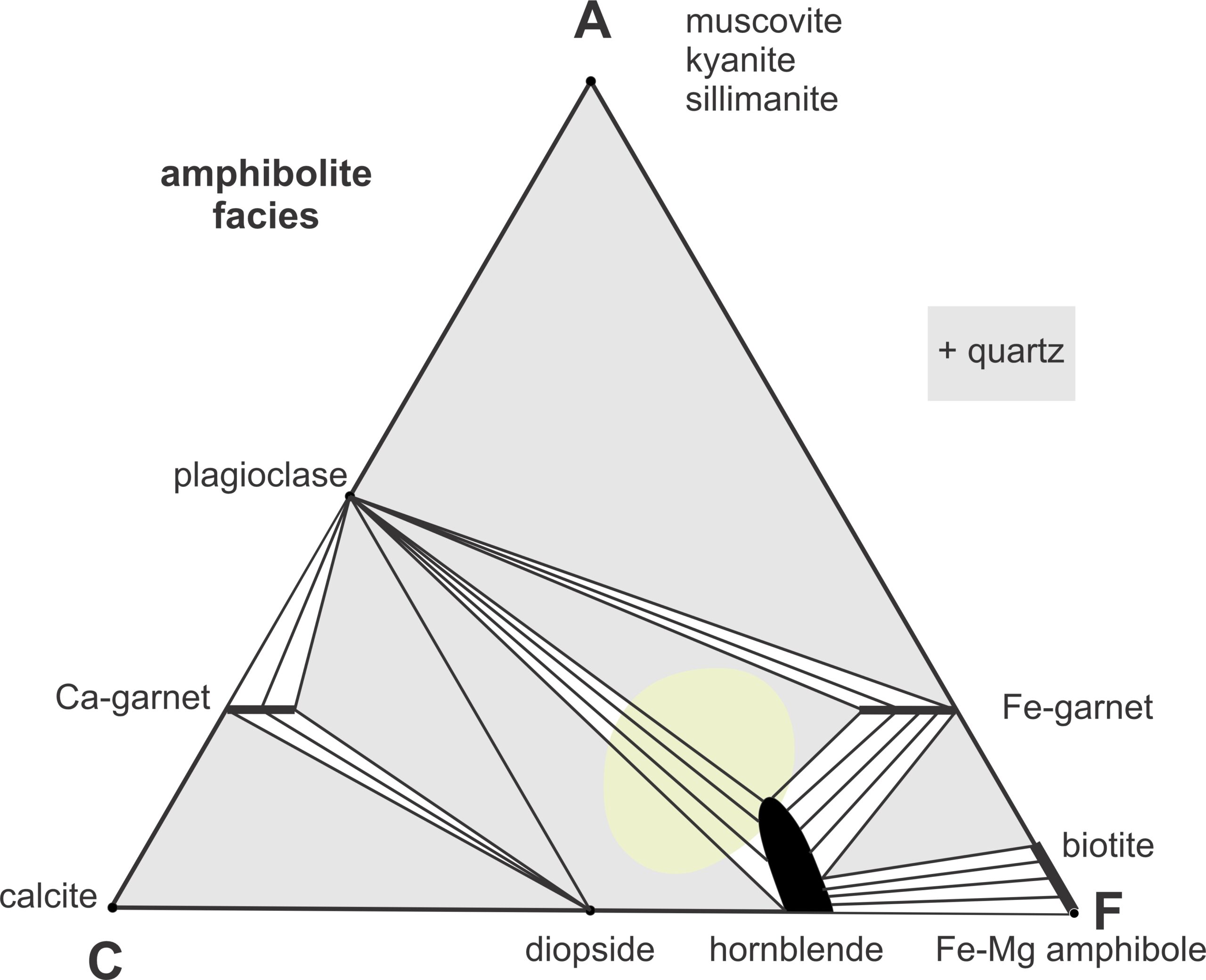
The transition from the greenschist facies to the amphibolite facies occurs at around 500 oC. The most noticeable change is the appearance of hornblende instead of actinolite. So, green, generally bladed, amphibole (actinolite) gives way to more blocky black amphibole (hornblende). Simultaneously, plagioclase gains calcium (mostly due to the disappearance of epidote) and is no longer albite. Many amphibolite facies metabasites appear to contain only macroscopic white plagioclase and black hornblende. They often have a massive structure; but gneissic foliation is sometimes present. Small to significant amounts of almandine (Fe-garnet) may be present, and in relatively aluminum-poor rocks, diopside may appear. Figures 13.2 and 13.16 contain photos of typical banded amphibolite, but foliation may be entirely absent. Figure 13.17 is an outcrop of dark non-foliated amphibolite that contains large garnets. Figure 13.18 is a closeup of the same rock. Look closely at Figure 13.18 and you can see red garnets intermingled with the hornblende and plagioclase.
13.4.4 The Granulite Facies
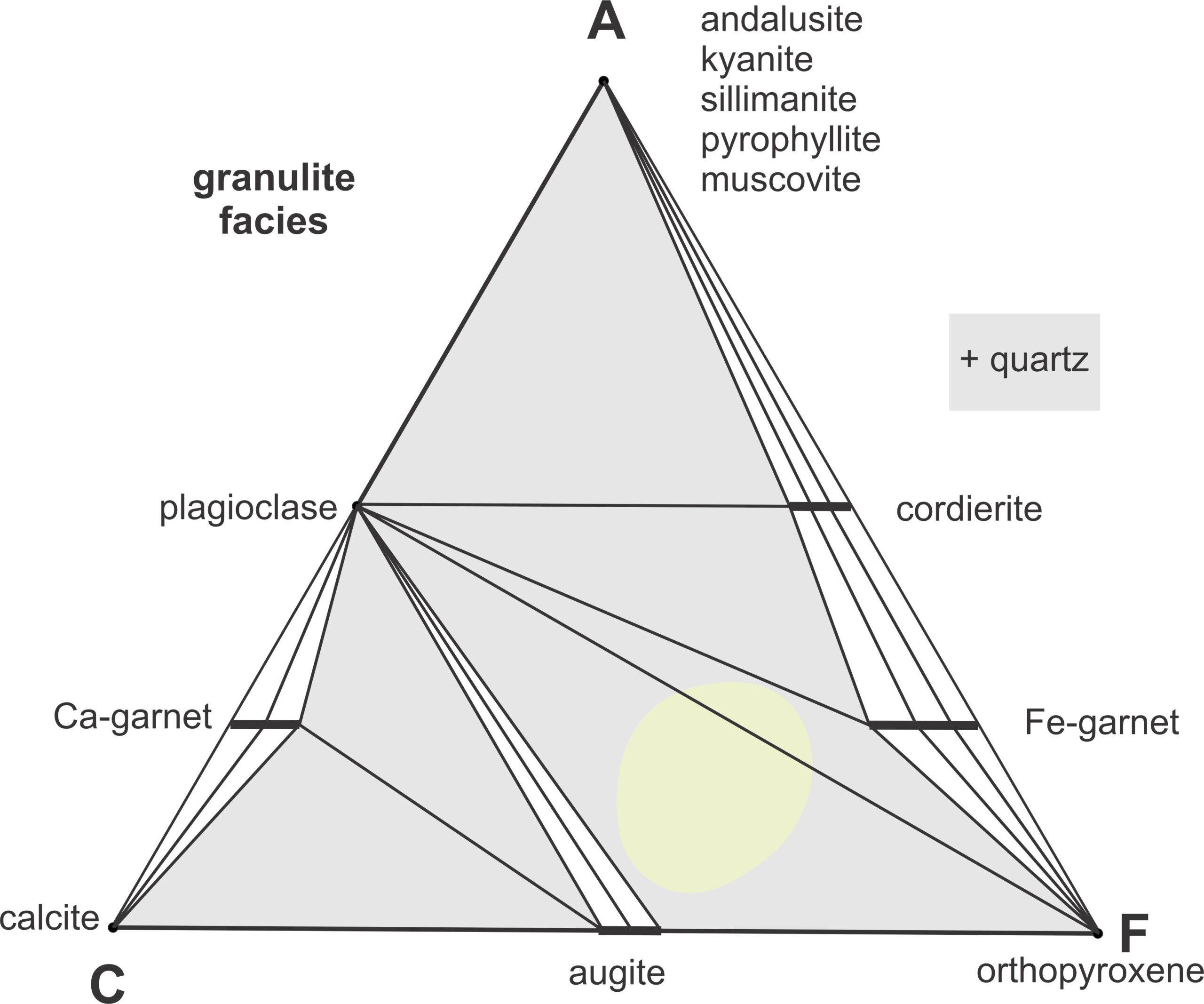
Granulite facies metabasites contain pyroxene and plagioclase with accessory quartz, garnet, oxides, and sometimes hornblende. Both orthopyroxene and clinopyroxene may be present, singly or together. The pyroxenes define granulite facies metamorphism of mafic rocks. Textures are generally granoblastic but some granulites exhibit gneissic banding. The minerals in granulites are the same minerals that make up most gabbros – but gabbros have igneous textures, often with tabular plagioclase and finer-grained pyroxenes between feldspar grains. Mafic granulites, in contrast, have a more equigranular mosaic texture. Hornblende and biotite are common in lower-temperature granulites, but hydrous minerals are absent from higher-temperature rocks . Figure 13.19 depicts the most typical mineral assemblages are augite-orthopyroxene-plagioclase and garnet-orthopyroxene-plagioclase, either with quartz.
The photo in Figure 13.20 is a somewhat weathered piece of granulite from a city park in Rio de Janeiro. We easily identify white plagioclase but, as is common for many granulites, identifying the pyroxene requires a thin section. Figure 13.21 is a garnet granulite from China. Figure 13.22 shows a vein that is rich in garnet, clinopyroxene, and plagioclase, cutting through a granulite outcrop. Garnet with clinopyroxene,and plagioclase is a common assemblage in granulite-facies rocks.
13.5 Low-Pressure Facies
Rocks of the low-pressure hornfels and sanidinite facies are generally very hard and fine-grained with no discernible metamorphic fabric. These rocks form when intruding magmas heat crustal rocks that are very near the surface and, thus, the rocks undergo contact metamorphism. Contact metamorphism that occurs at some depth in Earth grades into regional metamorphism and may produce rocks of the greenschist, amphibolite, or granulite facies. Thus, very low pressure Buchan metamorphism may be indistinguishable from hornfels facies metamorphism.
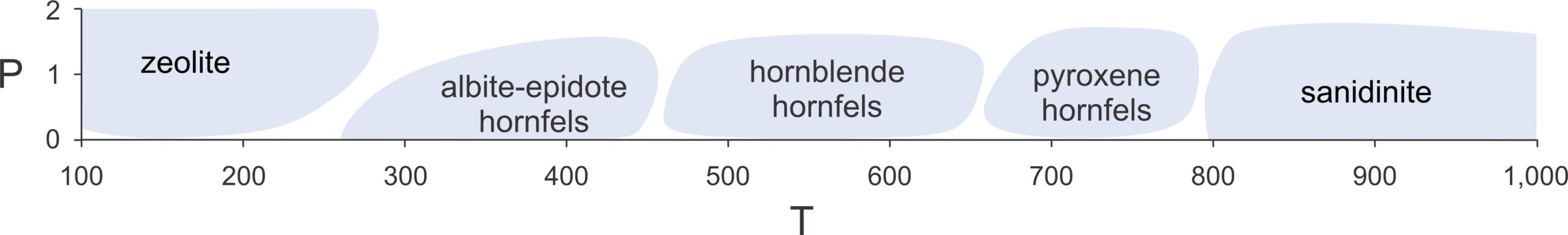

Petrologists often divide the hornfels facies into the albite-epidote hornfels facies (low grade), the hornblende hornfels facies (medium grade), and the pyroxene hornfels facies (higher grade) (Figure 13.23). The different facies are the results of contact metamorphism at different temperatures. In a field area that contains regional variations in metamorphic grade, we can map zones of these facies based on epidote, hornblende, and pyroxene occurrences in different places. At the highest temperatures before melting, contact metamorphism may take place at sanidinite facies conditions. The name of this facies derives from the high-temperature K-feldspar polymorph sanidine that may form in some contact metamorphic rocks. Metabasites metamorphosed under sanidinite facies conditions are quite rare and often contain unusual minerals. Some sanidinite facies rocks undergo partial melting and may contain glass.


Figure 13.24 is a photo of an outcrop of hornfels from western Ontario. This outcrop is typical, containing a fine-grained dark-colored rock with no macroscopic mineral grains. Most hornfels have uniform color like the sample in Figure 13.25, but Figure 10.2 was a photo of a banded hornfels from Novosibirsk, Russia. Some hornfels, howver, contain visible crystals. For example, the hornfels in Figure 13.26 contains (highly altered) porphyroblasts of cordierite. This hornfels, however, does not have the composition of a mafic igneous rock, like the most common hornfels do.
13.6 High-Pressure Facies
High P/T metamorphism (Figure 13.4) is associated with subduction zones where descending cold and wet lithosphere cools the mantle below. Rock is a good insulator, so temperatures in a subducting slab increase only slowly as the slab descends. Consequently, temperatures are not as great as they would be otherwise and metamorphism follows a high P/T path. So, in these environments, metamorphism begins with the zeolite and prehnite-pumpellyite facies, and subsequently may continue to the blueschist and eclogite facies (Figure 13.4). Blueschist- and eclogite-facies rocks are relatively rare at Earth’s surface, in large part because it requires special conditions to bring them to the surface from the depths where they formed. Additionally, most blueschist and eclogite occurrences are in allocthonous (unrelated to rocks around them) blocks or slivers of limited in size. Their limited and discontinuous occurrences reflect their uplift and exhumation as parts of accretionary wedges associated with subduction.
13.6.1 Blueschists
The kind of rock that forms under blueschist or eclogite facies conditions depends on the protolith. The rock type called blueschist forms when basalt and other mafic compositions reach blueschist facies pressures and temperatures. At those conditions, plagioclase becomes unstable, and the albite (Na) component reacts to make Na-rich amphibole and pyroxene. Consequently, blueschists contain glaucophane, a distinctive blue Na-rich amphibole (but despite their name, blueschists do not display schistosity). And blueschists and eclogites also commonly contain Na-rich pyroxene. We call such pyroxenes jadeite if they are close to NaAl2SiO6 in composition, and omphacite if they contain significant amounts of other components. As plagioclase breaks down, the anorthite (Ca) component reacts to form calcic pyroxene, amphibole, or epidote.
The photos in Figures 13.27, 13.28, and 13.29 are examples of blueschists. The rock from Greece in Figure 13.27 contains porphyroblasts of light-green blocky lawsonite, a high-pressure mineral with composition equivalent to hydrated anorthite. Figure 13.28 is a photo of the beach just north of Jenner, California. This beach contains spectacular outcrops of blueschists . Figure 13.29 is a sample from the beach that contains blue glaucophane, green omphacite (clinopyroxene), and red garnet. Besides the minerals seen in these photos, most blueschists contain epidote, titanite, and other accessory minerals.
13.6.2 Eclogites
Figure 13.30 (below) is an eclogite from the Nordfjord region of western Norway. Eclogites, by definition contain red garnet and green omphacite (Na-pyroxene) – these minerals can be seen in the Nordfjord eclogite. Figure 13.31 is a photo of a less common kind of eclogite – a kyanite eclogite from Slovenia. The kyanite appears as thin blue bladed crystals in the sea of garnet and omphacite. Eclogite facies rocks have variable compositions and appearances, but the rock type called eclogite forms when mafic rocks reach eclogite facies conditions. Eclogites are typically quite coarse-grained and often have a red-green Christmas-tree coloration/appearance, as seen in Figure 13.30.
Eclogite samples we find in outcrops today originated at great depth within Earth and the minerals within them are unstable at Earth’s surface. Consequently, many eclogites show signs of retrograde metamorphism. Some of the rocks on Jenner Beach (seen in Figures 13.28 and 13.29) appear to be part way between eclogite and blueschist – suggesting they originally formed under eclogite facies conditions and retrogressed as temperature and pressure decreased and moved into blueschist-facies conditions (see Figure 13.3). Figure 13.32 is a photo of a Jenner rock showing this transition. The greenish areas with red-brown garnet are relict eclogite but the eclogite has mostly been replaced by blueschist.
13.7 Ultramafic Rocks
13.7.1 Ophiolites, Serpentinites, and Metaperidotites
Earth’s mantle is made of ultramafic rocks. Peridotites, including mostly lherzolite, harzburgite, and dunite, dominate. At mid-ocean ridges, these ultramafic rocks rise to fill space created when plates diverge. This leads to decompression melting, creating mafic magmas that crystallize in the upper part of the lithosphere as gabbros, or that erupt as seafloor basalts.
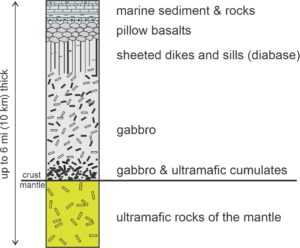
Overall, the oceanic lithosphere consists of a thin part of the uppermost mantle (ultramafic), overlain by mafic and ultramafic crust, in turn overlain by thin layers of seafloor sediments. The lithosphere cools and becomes denser during seafloor spreading, and so sinks. Thus, the youngest seafloor and highest elevations are at the ridges, and the oldest seafloor and lowest elevations at ocean margins (Figure 3.21). The oceanic lithosphere may not be the same composition and structure everywhere, but all evidence suggests that there are some standard components. The evidence comes from many sources including drill core, seismic studies, laboratory experiments, grab sampling from the ocean floor, and dredging. The best information, however, may come from studies of ophiolites. The term ophiolite derives from the Greek words ophio (snake) and lite (stone), referring to the commonly green color of the rocks that make up ophiolites.
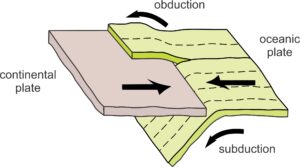
13.33 Obduction and subduction occurring at a subduction zone
Where oceanic and continental plates converge, oceanic lithosphere generally subducts beneath the continent. Sometimes, however, pieces of oceanic lithosphere are scraped off and added to a continent. We call this process obduction (Figure 13.33). Because of obduction, ultramafic rocks, formerly pieces of the mantle, can be found in ophiolite complexes. These complexes are wedges of ocean crust and mantle, now exposed in outcrops at the surface, that were thrust onto continental margins above subduction zones. Ophiolites are very important to geologists because they provide direct evidence for the nature of Earth’s oceanic crust and upper mantle. Although there are many ophiolites around the world, most are small or very fragmented. Table 13.2 lists some of the best known and studied ones. Most ophiolites are 10s or 100s of millions of years old because the processes of sea-floor spreading and obduction are slow. The Macquarie Island Ophiolite, the youngest known, is still more than 10 million years old.
| Table 13.2 Some of the Many Well-Known and Most Studied Ophiolite Complexes | ||
| ophiolite | mean age | notes |
| Coast Range Ophiolite (California) |
155 million years (Jurassic) | many scattered outcrops from Santa Barbara north to San Francisco |
| Semail Ophiolite (Oman and the United Arab Emirates) |
95 million years (Cretaceous) | large and very well exposed |
| Troodos Ophiolite (Cyprus) |
92 million years (Cretaceous) | especially well studied due to economical copper deposits associated with the ophiolite |
| Macquarie Island Ophiolite (Tasmania, Australia) |
10-40 million years (Cenozoic) | named a UNESCO World Heritage Site in 1997 |
| Bay of Islands Ophiolite (Newfoundland) |
485 million years (Ordovician) | well exposed nearly complete ophiolite sequence; named a UNESCO World Heritage Site in 1987 |
| Zambales ophiolites (Philippines) |
45 million years (Cenozoic) | several ophiolites of about the same age |
Every ophiolite provides a partial cross section of the oceanic lithosphere. When geologists combined information from many ophiolites with other evidence, a standardized model of the oceanic lithosphere, shown in Figure 13.34, emerged. A “complete” ophiolite would include all the layers of rock shown in the figure. These layers, which correspond to sediments and igneous rocks created by seafloor spreading, make up a cross section of the oceanic lithosphere. At its top, muds and other debris typically overlie hard rock. These sediments, which increase in thickness from mid-ocean ridges to ocean margins, may eventually lithify to form shale or chert. A layer of basalt, often containing pillow lavas, worm-like bodies formed during submarine eruptions, underlies the sediments. These basalts, like many ocean floor basalts, are commonly highly altered by interaction with seawater. Figure 13.25 is a photo of pillow basalts that formed on the ocean floor but are now exposed in an ophiolite in Oman.
|
|

Magmas that create ocean-floor basalts rise from magma chambers below, following fractures and creating vertical, parallel, mafic dikes. The many dikes produce a sheeted dike complex beneath the basalts. At still greater depth, a thick layer of gabbro is the remains of once liquid basaltic magma chambers (see Figure 13.34). The gabbro layer, accounting for most of the oceanic crust by volume, typically contains mafic to ultramafic cumulates in its lowest levels. Figure 13.36 is a photo of gabbro layers in the Troodos ophiolite – the browner layers contain significant amounts of orthopyroxene compared with the lighter colored layers. Peridotites (mainly harzburgites and lherzolites) of the oceanic mantle can be found beneath the gabbro layer.
The peridotites in the oceanic lithosphere are very high-temperature rocks and consequently the minerals they contain are unstable under normal crustal conditions. High-temperature minerals are especially unstable in the presence of water. So when uplifted to become part of the oceanic lithosphere, original olivine and pyroxenes typically are metamorphosed by seawater to produce a variety of different hydrous minerals. Further hydration also occurs during tectonism associated with subduction zones.

During continental collision and obduction, ophiolites may become incorporated into mountain belts to become bodies of rock we call alpine peridotites. These peridotite bodies range from small slivers to large plutons. Often the peridotites are dismembered pieces – all that remain of once more complete oceanic lithosphere. Alpine peridotites typically contain serpentinized rocks. Figure 13.37 shows an outcrop of folded serpentinite from the Tux Alps in central Austria. We saw other photos of serpenentinite oucrops in Figure 2.25, Figure 7.41, Figures 9.24 and 9.25, and Figure 11.13. Sometimes alpine peridotites also include more pristine ultramafic rock, gabbro, basalt, or marine sediments.
Serpentinization of a typical mantle peridotite produces a rock composed of serpentine and brucite. Less commonly if the original rock was more SiO2-rich than a typical peridotite, serpentinization may also produce talc. So, after serpentinization, an originally high-temperature rock contains low-temperature hydrous minerals. However, subsequent heating and metamorphism during orogenesis often leads to reequilibration as hydrous minerals break down and higher-temperature minerals once again form. So, although many of the ultramafic rocks we see probably were serpentinites at one time, they may contain any of a number of minerals. Table 13.3, below, lists the most common of these minerals.
| Table 13.3 Minerals, abbreviations, formulas | ||
| mineral | abbrev. | formula |
| anthophyllite antigorite brucite chrysotile diopside enstatite forsterite periclase quartz talc tremolite |
Ath Atg Br Chr Di En Fo Pe Qz Tc Tr |
Mg7Si8O22(OH)2 Mg3Si2O5(OH)4 Mg(OH)2 Mg3Si2O5(OH)4 CaMgSi2O6 Mg2Si2O6 Mg2SiO4 MgO SiO2 Mg3Si4O10(OH)2 Ca2Mg5Si8O22(OH)2 |
The principal components in ultramafic rocks are CaO, MgO, and SiO2. Besides these components, several weight% of FeO and Al2O3 may be present, but these components do not significantly affect the stable mineral assemblages at low to moderate temperatures. So, the main minerals are Mg-silicates and Ca-Mg silicates. Thus the reactions that affect metaserpentinites can be modeled with a relatively simple four-component system: CaO-MgO-SiO2-H2O.
In pristine ultramafic rocks, olivine (forsterite) and pyroxenes (enstatite and diopside) dominate. In serpentinites and other altered rocks, these primary minerals may be gone. The rocks may be entirely serpentine (antigorite or chrysotile), brucite, or talc. Antigorite and chrysotile are the most common forms of serpentine. Although listed with the same formulas in Table 13.3, their composition differ slightly.
13.7.2 The CaO-MgO-SiO2-H2O System
Figure 13.38 (below) is a petrogenetic grid for the CaO-MgO-SiO2-H2O system. The diagram includes all possible reactions involving the 10 most common minerals in the system. Chemographic triangles for each field, projected from H2O, provide graphical views of the stable mineral assemblages. However, because diopside is the most Ca-rich phase in the system, we have used CaMgSi2O6 (diopside composition) instead of CaO for one of the components. (This spreads out the points in the triangles.)
For the most part, peridotites have an overall composition that plots near the point for antigorite (Atg). Most of the reactions in Figure 13.38 are degenerate (involving 4 phases) or doubly degenerate (involving 3 phases). Of the minerals being considered, only diopside and tremolite contain CaO. So, the two 5-phase reactions that involve diopside have tremolite on the opposite of the equal sign.
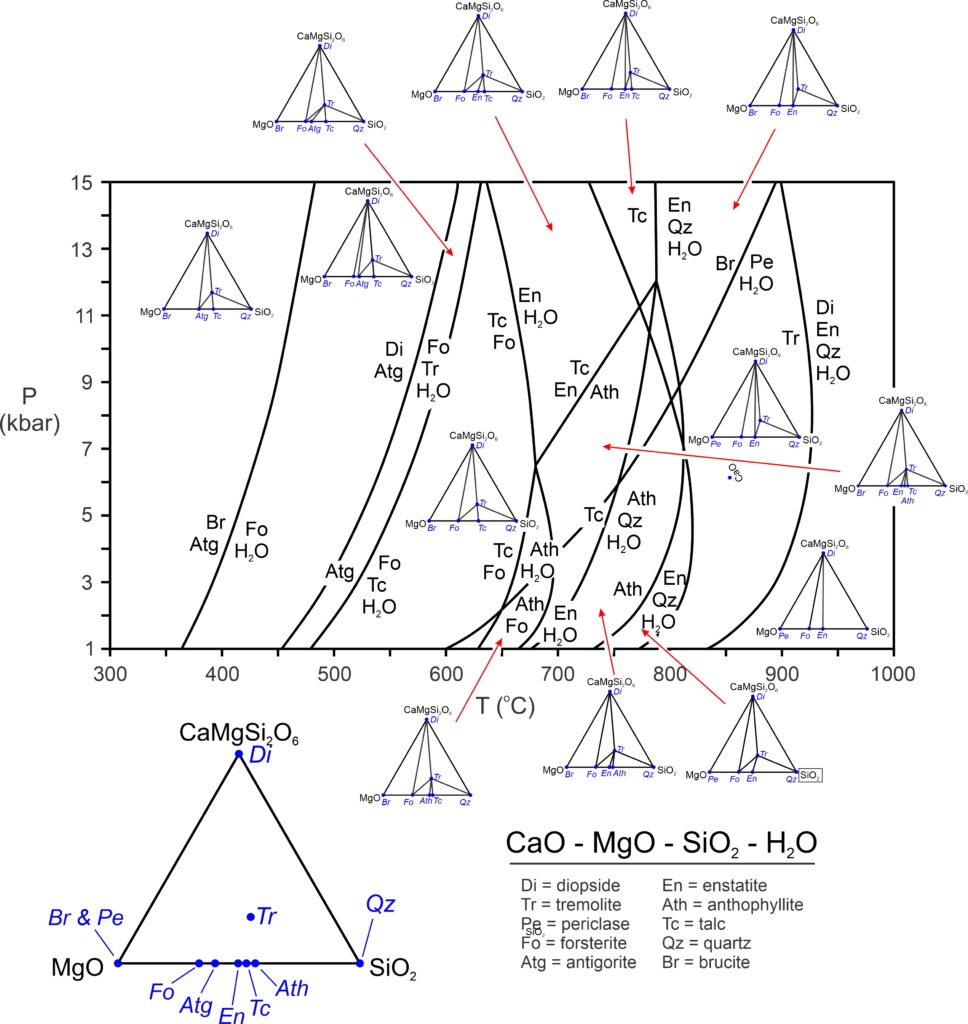
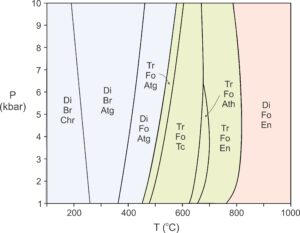
As pointed out in Chapter 11 (Section 11.6), the reactions that appear in a phase diagram like Figure 13.38 do not all apply to all rocks. The specific reactions that may occur depend on rock composition. Figure 13.39, a pseudosection based on the petrogenetic grid above, shows the stable mineral assemblages for typical metaserpentinite compositions. The reactions involved, and thus the fields, are mostly vertical. So, the mineral assemblages are good indicators of metamorphic temperature but not of metamorphic pressure. The phase rule tells us that for a four-component system, the stable assemblages between reaction curves contain three minerals (plus H2O, which is not labeled on this diagram). That is what we see in Figure 13.39.
The mineral relationships in Figure 13.39 follow the same trends that we see for all mineral systems. During progressive metamorphism, H2O-bearing minerals break down. With increasing temperature, serpentinites lose the hydrous minerals serpentine (chrysotile and antigorite) and brucite, and eventually amphiboles (tremolite and anthophyllite). Only (anhydrous) diopside, enstatite, and forsterite remain at high temperature. These are the same primary minerals that form when ultramafic rocks crystallize from a magma. Although Figure 13.38 depicted reactions involving talc, talc is not stable anywhere in Figure 13.39 because talc-bearing rocks contain more SiO2 than typical peridotites.
The different mineral assemblages shown in Figure 13.39 are not unique to different metamorphic facies, but there is a good correlation between facies and assemblages based on the presence or absence of amphibole (tremolite or anthophyllite). The low temperature part of this diagram (blue) corresponds to the zeolite, prehnite-pumpellyite, and greenschist facies (at low pressure) and to the blueschist facies (at high pressure). Assemblages in the middle part of the diagram (green) that contain tremolite or anthophyllite (both amphiboles) equilibrated in the amphibolite facies. And the diopside-forsterite-enstatite assemblage (reddish field) represent the granulite facies.
13.7.3 The Effects of Other Components
13.7.3.1 FeO Content
The diagrams in Figures 13.38 and 13.39 are for the four-component CaO-MgO-SiO2-H2O system. Yet peridotites and serpentinites may contain other components. For example, ultramafic rocks may have several weight % FeO or Al2O3. They may also contain CO2. It is worth considering what the effects of these three additional components might be. Other components, generally present in very small amounts, have insignificant effects on stable mineral assemblages.
Olivine, pyroxene, serpentine, and amphiboles always contain some iron. Although there is much more magnesium than iron, the iron content has an effect on the temperatures at which the reactions in Figures 13.38 and 13.39 occur. This happens because greater amounts of iron extend the stability of anthophyllite and enstatite, and greater amounts of magnesium extend the stability of talc and diopside. Essentially, when we consider the effects of iron, we are considering the effects of an additional component. This means we have an additional degree of freedom and the reactions shown in the diagrams above become divariant and no longer plot as lines. They plot as vertical bands, instead. The effects of variable iron and magnesium content are not, however, great. Frost and Frost (2019) calculated that the reactions may shift up or down by a few 10s of degrees depending on the compositions of the minerals involved. Iron may also have another effect: metaperidotites that contain greater amounts of iron may contain more magnetite or spinel than rocks with less iron.
13.7.3.2 Al2O3 Content
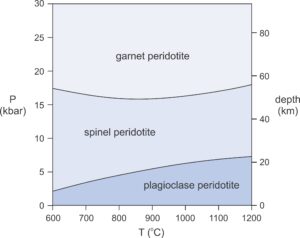
Ultramafic rocks sometimes contain up to 3 or 4 wt% Al2O3. If sufficient alumina is present, chlorite will be present in rocks that equilibrate below 700 or 800 oC, depending on pressure. At higher temperatures, where most peridotites contain olivine, orthopyroxene, and clinopyroxene, aluminum is a component in pyroxene. It may also lead to plagioclase, spinel, or garnet instead of chlorite. These minerals, rarely present in significant amounts, are good indicators of metamorphic pressure.
|
|
|
Plagioclase peridotites are stable at low pressures corresponding to shallow depths in Earth (Figure 13.40). Spinel peridotites are stable at somewhat greater pressures. Garnet peridotites are stable at even greater pressures, generally greater than 16 kbar, equivalent to depths of more than 50 km in Earth. Figure 13.41 is a photo of a garnet peridotite from Switzerland. Plagioclase peridotites are quite rare compared to the other two types.
Besides occurrences in alpine peridotites, many samples of spinel peridotite and garnet peridotite are in xenoliths carried to Earth’s surface by basaltic magmas or by kimberlites. The xenoliths provide the only way for us to look directly at the rocks from Earth’s lower crust and upper mantle, so they give us information that otherwise would be unavailable. Xenoliths vary somewhat in mineralogy. Some are garnet or spinel peridotites, but most are lherzolites, harzburgites, dunites, or clinopyroxenites.
13.7.3.3 CO2 Content

Typical serpentinites contain veins of carbonate minerals. The veins are, for the most part, secondary, developing long after the rock formed. Sometimes, however, fluids present during serpentinizaton, or during subsequent metamorphism or alteration, contain sufficient CO2 so that magnesite (Mg-carbonate) or dolomite (CaMg-carbonate) are stabilized. In rocks that equilibrated at low to moderate temperature, these carbonate minerals may be present instead of the Ca-Mg silicates diopside and tremolite. Figure 13.42 shows a polished slab of a rock that contains white magnesite and green serpentine.
| Chapter 16 Section 16.3.2 contains many photos of metamorphosed mafic and ultramafic rocks in hand specimen and in thin section. If you want to know what they look like, go there. |
● Figure CreditsUncredited graphics/photos came from the authors and other primary contributors to this book. 13.0 (opening photo) Metabasite outcrop, Stacy Phillips, travelinggeologist.com |