KEY CONCEPTS
- Although Earth’s volcanic rocks have variable compositions, and contain many different elements, silicon and oxygen dominate most magmas.
- Petrologists generally report igneous rock compositions by listing oxide weight percentages. Silicon and oxygen dominate most magmas, with silica (SiO2) comprising 40-80% of overall composition.
- At a fundamental level, we use the general terms ultramafic, mafic, intermediate, and silicic to describe rocks and magmas ranging from low silica-content to high silica-content. Silica content has a major effect on magma properties.
- Most lavas have temperatures between 700 and 1,300 oC. Silicic lavas are at the low end of this range, and mafic lavas at the high end.
- Magmas may contain up to 7 wt% volatiles, typically water vapor, carbon dioxide, and sulfur gases. Gas pressure is the main force causing violent volcanic eruptions.
- Most magmas originate in the mantle. Exceptions include some silicic magmas in continental regions.
- Several different mechanism may cause rock to melt to produce magma. The most significant of these are decompression melting that occurs at mid-ocean ridges, and flux melting that occurs at subduction zones.
- Magmas are naturally buoyant, and a race to the surface determines if rising magmas will erupt or if they will crystallize at depth.
- Magmas may not have the same composition as their parent rocks, and magmas evolve and change composition after they first form.
- Petrologists and geochemists use magma chemistry to trace paths of magma evolution.
3.1 Volcanism in Yellowstone National Park
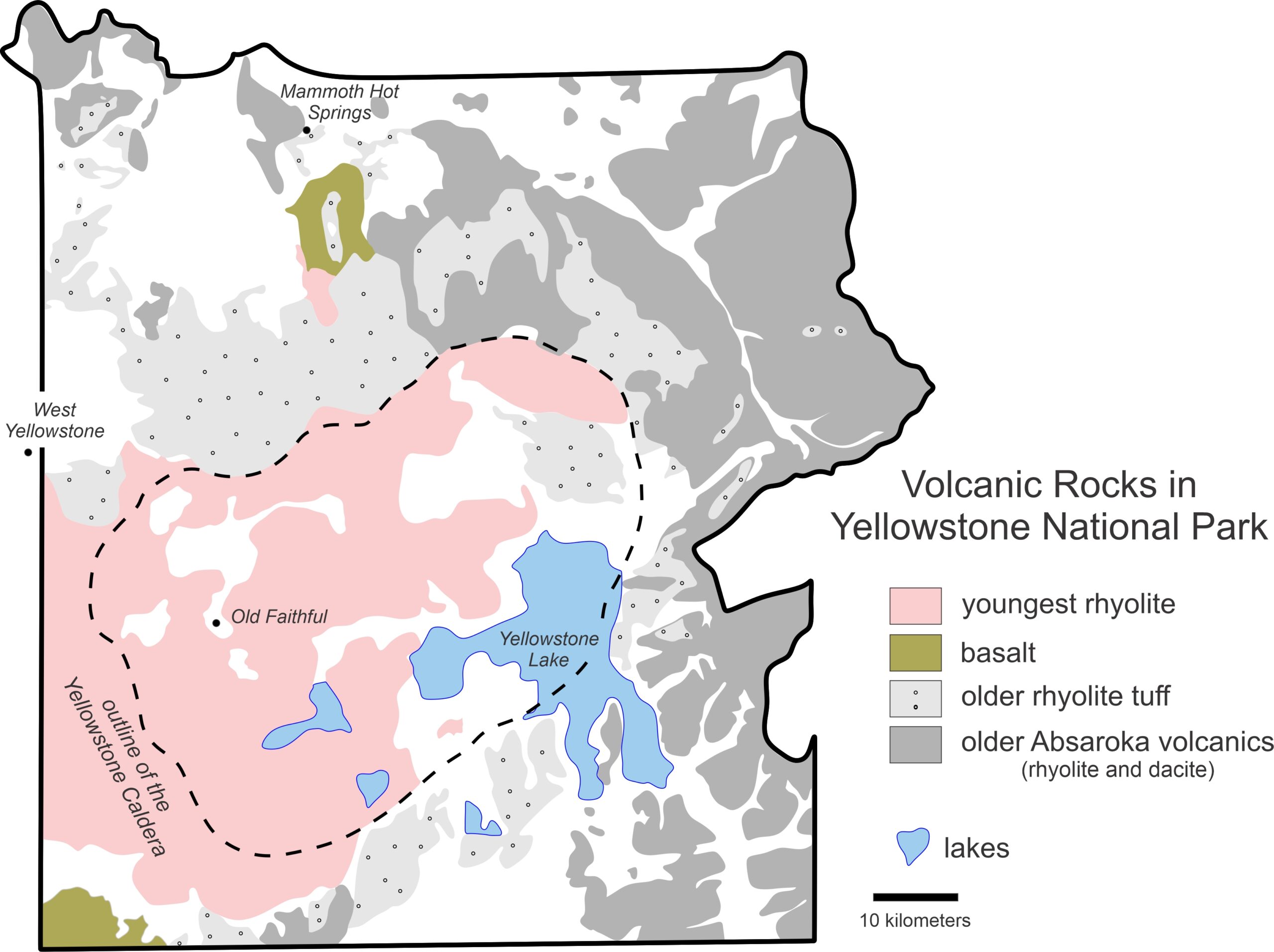
Although Earth’s volcanic rocks have variable compositions and contain many different elements, silicon and oxygen dominate most magmas. The most common magmas range from rhyolite, typically containing 70-77 wt% silica (SiO2, silicon oxide), to basalt, typified by 45-55 wt% silica. Yellowstone, America’s first national park, contains both high-silica and lower-silica igneous rocks. Most of the Yellowstone eruptions yielded rhyolite tuffs and flows, but lesser amounts of basalt, containing much less SiO2, are also present. Figure 3.1 shows the distribution of different kinds of volcanic rocks exposed in Yellowstone today.

The Absaroka Volcanic Field, in dark gray in Figure 3.1, dominates the eastern part of the Yellowstone region. The Absaroka rocks accumulated 53-43 million years ago, during the Eocene Epoch. The youngest Yellowstone volcanic rocks are much younger, and accumulated during three major periods of volcanism that included some of the largest eruptions ever on Earth. The first of these eruptions was 2.1 million years ago, in the early Pleistocene; the last of the eruptions was relatively minor and ended about 70,000 years ago. The huge, violent events threw much debris into the air, Yellowstone ash settled in layers over much of the western United States and circled the globe. In the Yellowstone region, soft ash, pumice, and other rock-fragment material accumulated and ultimately became harder rocks. Nowhere are these rocks better exposed than in the Grand Canyon of the Yellowstone, seen in Figure 3.2.
The Grand Canyon of the Yellowstone is a deeply eroded chasm exposing volcanic rocks formed from volcanic ash deposits and lava flows created about 500,000 years ago. The lava and ash formed from silicic (rhyolitic) magma. Unfortunately, for geologists, Yellowstone is an active geothermal area, and warm flowing groundwaters have altered the original igneous rocks. Many of their original features have been obscured, but oxidation during the alteration has produced colorful landscapes that thrill visitors and inspire photographers and artists.

Besides the rhyolites exposed in Yellowstone’s Grand Canyon, relatively recent Yellowstone volcanic activity has produced basalt (Figure 3.3). The basaltic eruptions occurred as horizontal flows that solidified and today appear as hard, jointed cliffs with vertical columns. The magma that produced these basalts had a distinctly different composition from the more common rhyolitic rocks found in the region. The columns seen in Figure 3.3 formed as lava cooled and shrank, much the same way that mud cracks form when a muddy puddle of water dries up. Although most well-formed columns are associated with basalt flows, in Yellowstone columns also developed in rhyolitic ash deposits.
Geologists call the occurrence of rhyolite and basalt, without rocks of intermediate composition, bimodal volcanism. Bimodal volcanism is often associated with rifts in Earth’s continental crust that, given enough time, may become the sites of new oceans. Bimodal volcanism is also commonly associated with hot spots where upwelling heat rises from the mantle as a thermal plume that can cause melting and so produce volcanism. Both rifts and a major hot spot characterize the Yellowstone region.
The hot spot and the associated rift beneath Yellowstone delivered heat to the region. One result was mafic (basaltic) magma, a small amount of which eventually reached the surface. The mafic magma originated by partial melting of the uppermost portions of underlying mantle – at least 30 kilometers beneath the surface. Melting occurred, in part, because of extra heat delivered by the hot spot. It also occurred because pressure decreased as mantle material moved toward the surface, and lower pressure makes it easier for geological materials to melt. Partial melting of the mantle is common and widespread, but most magmas that originate in the mantle do not make it to the surface. Instead, they solidify underground or remain molten in deep magma chambers. The small amounts that do make it to the surface pass through the crust quickly and may eventually produce basalt flows such as those exposed at Sheepeaters Cliff , seen above in Figure 3.3.
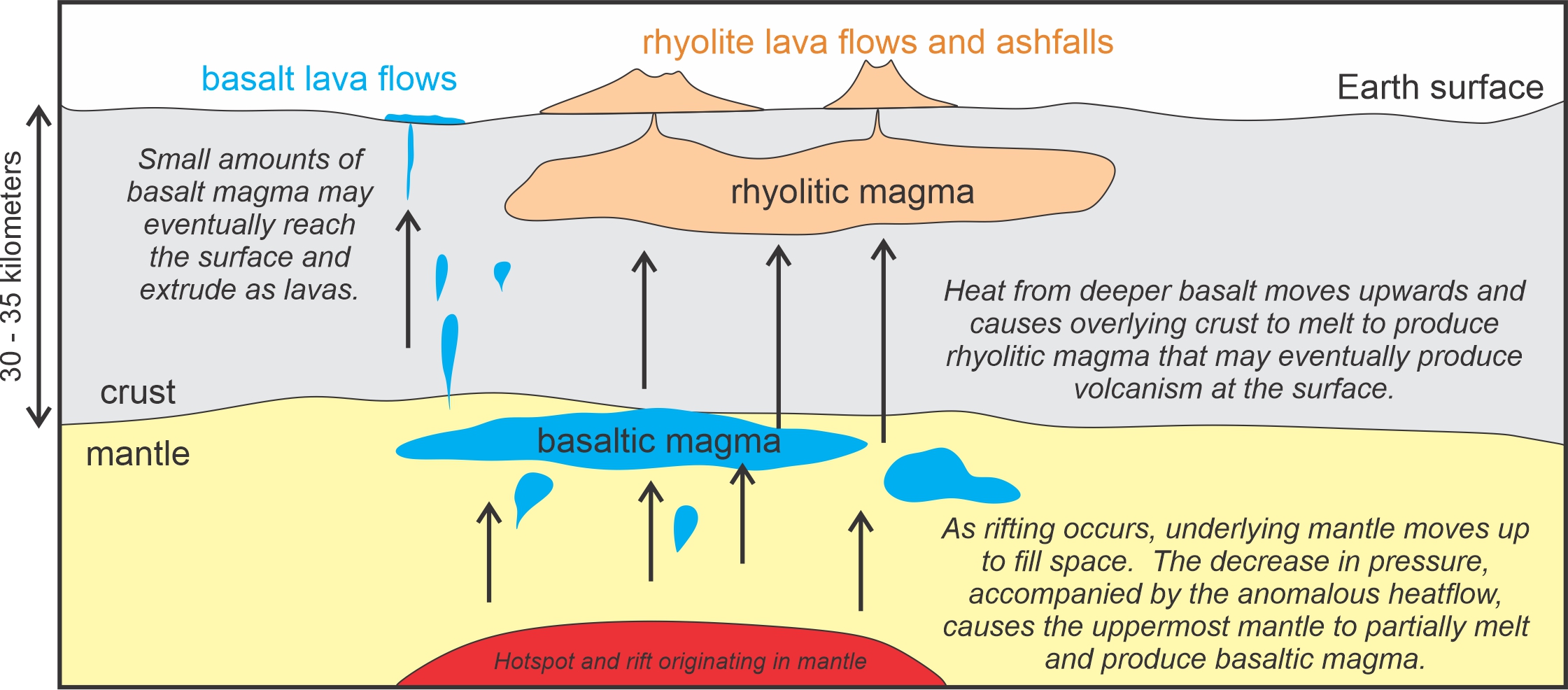
Most significantly, the basaltic magma beneath Yellowstone delivered heat to the crust. Most crustal material melts at lower temperature than does basalt and, consequently, rising basaltic magmas from the mantle can cause melting in overlying rocks. Whether the basalt made it to the surface or not, it is likely that lower crustal material melted to become rhyolitic magma. These are the magmas that have, historically, dominated Yellowstone eruptions (Figure 3.4). Other explanations for the rhyolitic magma in Yellowstone involve fractional crystallization of a basaltic parental magma, and crustal assimilation by an intermediate or basaltic magma. We discuss these processes further later in this chapter.
So, although found together, Yellowstone’s rhyolite and basalt (silicic and mafic) magmas most likely formed from magmas coming from two different parts of Earth and formed during different kinds of melting events (Figure 3.4). The basaltic magmas originated in the mantle, and the rhyolitic magmas originated in the crust. See also Figure 3.25, later in this chapter.
Geophysical studies have revealed large partially-melted silicic magma chambers under Yellowstone today, at depths as shallow as just a few kilometers. In the past, such chambers were the sources of the magmas associated with major eruptions. When the chambers emptied during Yellowstone’s major eruptions, the overlying crust collapsed, producing a depression known as a caldera (outlined by a dashed line in Figure 3.1). The present-day Yellowstone Caldera is a 4,000 square kilometer shallow depression that occupies about half of Yellowstone National Park. It formed after the Yellowstone eruption that occurred 640,000 years ago.
● Box 3.1 Geothermal Features in Yellowstone National ParkThe last major eruption at Yellowstone was 640,000 years ago. But magma can be found just a few kilometers beneath the surface today. That magma gives off a lot of heat. The heat produces very hot groundwater, often at temperatures that create steam. So, Yellowstone National Park contains many spectacular geothermal features, including hot springs, fumaroles, steam vents, mudpots, and geysers. Some of the most common geothermal features in Yellowstone are hot springs where rising hot water reaches the surface. Fumaroles and steam vents are holes or vents where steam is produced instead of water. Mudpots are hot spring or fumaroles where the water is especially sediment rich (muddy). Geysers, such as Castle Geyser in Figure 3.5) are hot springs that erupt periodically when steam pressure becomes high, causing an eruption of water and steam into the air. As eruptions continue, pressure decreases and the eruption eventually stops. Subsequently, pressure may build up and another eruption can occur. Hot water may contain a lot of dissolved carbonate. Travertine terraces form when that carbonate precipitates at the surface. Mammoth Hot Springs (Figure 3.6) is one of the best examples anywhere. These terraces are actively growing today. |
3.2 Magma Compositions
3.2.1 Similarities
The same elements dominate most magmas, and these elements are the same as those that make up most minerals. Eight of the nearly 120 known elements account for >98% of the compositions of common magmas: oxygen, silicon, aluminum, iron, calcium, sodium, potassium, and magnesium. Consequently these elements are the principle components of igneous rocks. Several other elements, including titanium, hydrogen, phosphorous, manganese, carbon, and sulfur are sometimes present in significant, but secondary amounts.
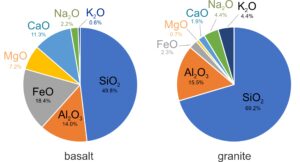
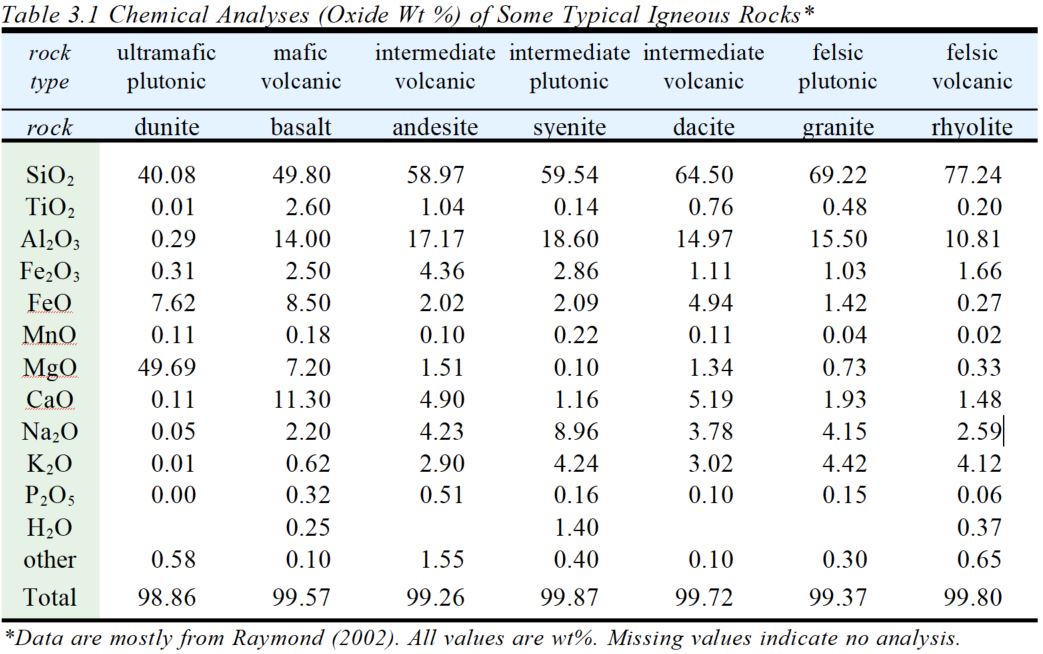
Petrologists generally report magma compositions by listing oxide weight percentages. Silicon and oxygen dominate most magmas, with silica (SiO2) comprising 40-80% of the overall composition. The pie charts in Figure 3.7 compare the compositions of typical basaltic (mafic) and granitic (silicic) magmas. The numbers are wt % values for the different oxides in both magmas. SiO2 and Al2O3 make up a large part of both mafic and silicic magmas. Mafic magmas, however, contain significantly more FeO, MgO, and CaO than silicic magmas, and silicic magmas contain more Na2O and K2O.
The pie charts above only included the 7 most abundant oxides. Magmas and rocks always contain other oxide too. Table 3.1 presents typical analyses, including some very minor components, of some common igneous rocks (and thus the compositions of common magmas). Most contain 6-8 oxides at levels greater than 1 wt %, and another half dozen that are present at smaller, but sometimes significant, ]]]]]]]]]]]]]]]]]]]]]]]]]]]]]]]]]]=levels.
We classify and group igneous rocks (and magmas) in many ways. At a fundamental level, we use the general terms ultramafic, mafic, intermediate, and silicic for rocks and magmas with low silica contents to high silica contents, respectively. The analyses in Table 3.1 include rocks with silica contents ranging from 40.08 wt% (ultramafic rocks) to 77.24 wt% (silicic rocks). Rocks and magmas with silica contents outside this range exist but are rare. Because of the high silica content in magmas, most minerals in igneous rocks are silicates.
(Note that the chemical oxides listed in Table 3.1 should not be confused with mineral formulas. That is, if an analysis shows that a rock contains 58.97 wt% SiO2, it does not mean that the rock contains 58.97 wt% quartz, even though quartz has composition SiO2. The silica (SiO2) in the rock may be distributed among a variety of silicate minerals that may or may not include quartz.)
3.2.2 Differences
Although the major element chemistries of most magmas, and thus the chemistries of most lavas and other igneous rocks, are similar, the relative amounts of different chemical components vary quite a bit. As just pointed out, silica (SiO2) content is, perhaps, the most important thing that sets magmas apart, but variations in other oxides are often important too. Some (alkalic) magmas are relatively rich in K2O and Na2O, for example, and the ratio FeO/MgO is variable too. Thus, magmas vary in composition and, accordingly, in their properties. So, too, do the lavas that form from magmas. For example, silicic lavas tend to be very viscous; intermediate-composition magmas have less viscosity, and mafic magmas are quite fluid. This is because silica can polymerize in melts and make them gooey – more silica (more silicic) means more polymerization and viscous magmas. In contrast, mafic lavas are often much hotter than rhyolitic lavas and quite fluid. Although all magma can flow down hill, basaltic (mafic) lavas move at a much faster rate than their silicic counterparts and sometimes move as lava “rivers.”

Mafic lavas often flow long distances before solidifying. But due to greater viscosity, silicic ones may not flow very far. Figure 3.8 (top) shows a lava river flowing from Mauna Loa Volcano in 1984. The surrounding dark and light gray material is all basalt. The darker material solidified within a few months of when this photo was taken and the other basalt is several years old. The bottom photo in Figure 3.8 shows a round mound of silicic material – called a silicic dome – in the summit crater of Colima Volcano in Mexico. This volcano is the most active volcano in Mexico and has a history of both explosive eruptions and quieter lava flows. The dome at its summit formed from gooey silicic lava that erupted in the crater bottom but was so viscous that it piled up instead of flowing outward. The dome disintegrated during later eruptions, after this photograph was taken, producing lavas that flowed down the sides of the volcano.
Adding a further complication, magmas are generally not entirely molten and, so, the distinction between magma and solid rock is not as sharp as we might expect. Different minerals crystallize at different temperatures, and consequently many magmas and lavas are mixtures of melt and solid (crystalline) components as they cool but before they are completely solid.

Although all glowing lavas appear very hot, they have a wide range of temperatures that depends on composition. Most lavas have temperatures between 700 and 1,300 oC. Silicic lavas are at the low end of this range, and mafic lavas at the high end. Rare carbonate-rich lavas called carbonatites, which do not contain significant amounts of SiO2, may be as cool as 600 oC. At the other end of the temperature spectrum, ultramafic lavas may have temperatures greater than 1,500 oC. Figure 3.9 shows the crater at the top of Ol Doinyo Lengai in Tanzania, an active volcano that produces carbonatite lavas. This is the only carbonatite volcano known to have erupted in the past 10,000 years. The cone shaped structure in this photo is where a lava fountain occurred and the white rock in much of the photo is solidified carbonate-rich lava.
3.2.3 Xenoliths
Magmas may contain xenoliths, fragments of rock in a magma. If a xenolith comprises a single mineral crystal, we call it a xenocryst. The two terms come from Greek words for “foreign rock” and “foreign crystal.” Some xenoliths and xenocrysts are products of partial magma crystallization and genetically related to the host magma. We call them autoliths or cognate xenoliths/xenocrysts. Other xenoliths, sometimes called exotic xenoliths, have a distinctly different origin than the magma that incorporates them. Typically, exotic xenoliths were plucked from surrounding solid country rock as a magma passed through. Exotic xenoliths give us samples and valuable chemical data about regions deep within Earth – information that we cannot obtain in any other way.

This photograph (Figure 3.10) shows a pyroxenite (exotic) xenolith in a volcanic rock from La Palma, one of the Canary Islands. Besides the xenolith, the rock contains visible phenocrysts of K-feldspar (tan colored) and lesser amounts of black biotite. The large (7 centimeters across) pyroxenite fragment is a piece of rock that was picked up deep in Earth as the magma moved toward the surface. The presence of the xenolith tells us that its melting point was higher than the temperature of the host volcanic rock.
3.2.4 Volatiles in Magmas

Magmas sometimes contain up to 7 wt% volatiles, which are gases dissolved in the molten material. Water vapor, carbon dioxide, and sulfur gases are typically present. Other volatiles, including chlorine or fluorine gases, may be present too. When magma is under pressure at depth in Earth, gases are simply minor components that dilute molten material. However, when magma moves toward Earth’s surface, pressure decreases, and the dissolved gases may form bubbles or separate from the magma completely and escape into the atmosphere. Commonly, however, some gas bubbles become trapped, forming vesicles (holes) and, eventually, a vesicular volcanic rock. This photograph (Figure 3.11) shows a colorful lizard on top of vesicular basalt on Lanzarote Island in the Canary Islands.

Sulfur in magmas is typically in the form of sulfur dioxide or hydrogen sulfide gases. The amount of sulfur varies from volcano to volcano. Lacking a volcanic eruption, sulfur-rich volcanic gases may reach the surface to form fumaroles, openings where volcanic gases are emitted for prolonged times. Some fumaroles, such as this one (Figure 3.12) in Hawaii, are associated with significant sulfur deposits because, when gases reach the surface, water vapor, carbon dioxide, and other volatiles escape into the air, leaving sulfur behind. Figure 3.13 shows centimeter-sized sulfur crystals at Kilauea in Hawaii.

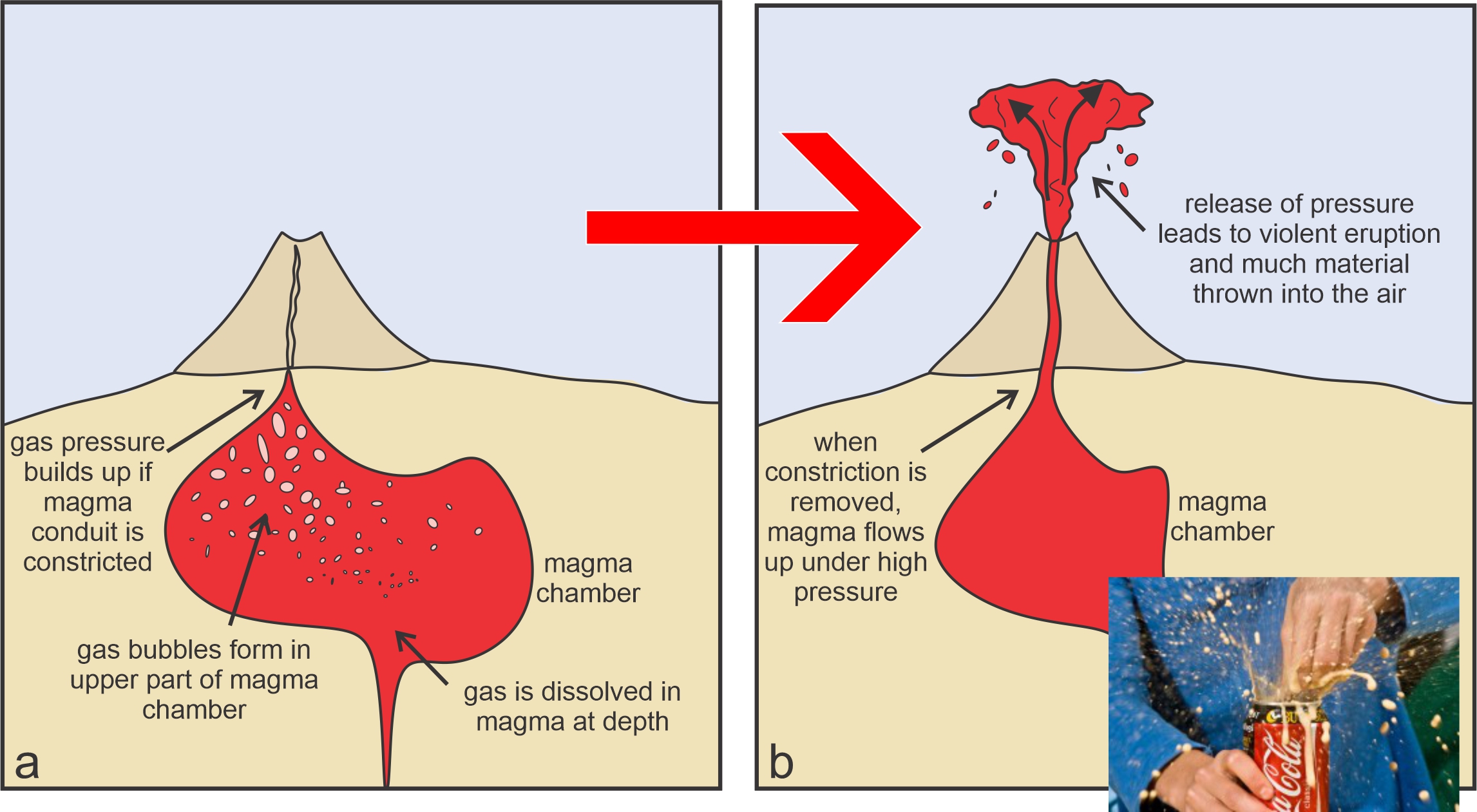
Gas pressure is a major force – actually the only major force – behind violent volcanic eruptions. Dissolved volatiles form bubbles as magma moves upward and confining pressure decreases (Figure 3.14a). The bubbles expand as pressure decreases with continued upward movement. The expanding gas not only provides gas pressure, but dilutes the magma, making it less dense and adding buoyancy that enhances upward movement. Constrictions in a magma conduit can help pressure build up. Eventually, if gas expansion and upward movement are fast enough, the result is an explosive eruption, much the same way that pop may explode out of a pop can if it is opened too quickly (Figure 3.14b). So, the main factor determining whether an eruption is explosive is the concentration of volatiles in a magma. Silicic magmas are much more likely to cause explosions because they are more viscous and so tend to hold on to bubbles. In contrast, when bubbles form in thin, more fluid, mafic magmas, the bubbles generally can escape.

Volcano (Guatemala, 2008), top; and the explosive Sarychev Volcano (Russia, 2009), bottom
Figure 3.15 contrasts the eruptions of two volcanoes. The 2008 eruption of Pacaya Volcano (top), in Guatemala, produced slow-moving lava flows. The 2009 eruption of Sarychev Volcano (bottom), in Russia’s Kuril Islands, was explosive and violent, throwing ash and larger debris more than a thousand meters into the air. Relatively tame volcanoes, such as Pacaya and also all of Hawaii’s volcanoes, are great tourist attractions – note the spectators in the top photo in Figure 3.15. Explosive volcanoes such as Sarychev, are extremely violent and pose huge risks to people and infrastructure up to many 10s of kilometers away from the site of the eruptions. The contrasting types of eruptions, discussed more fully in Chapters 4 and 5, are due primarily to differences in magma chemistries.
3.3 Magma Sources
3.3.1 Where Melting Occurs
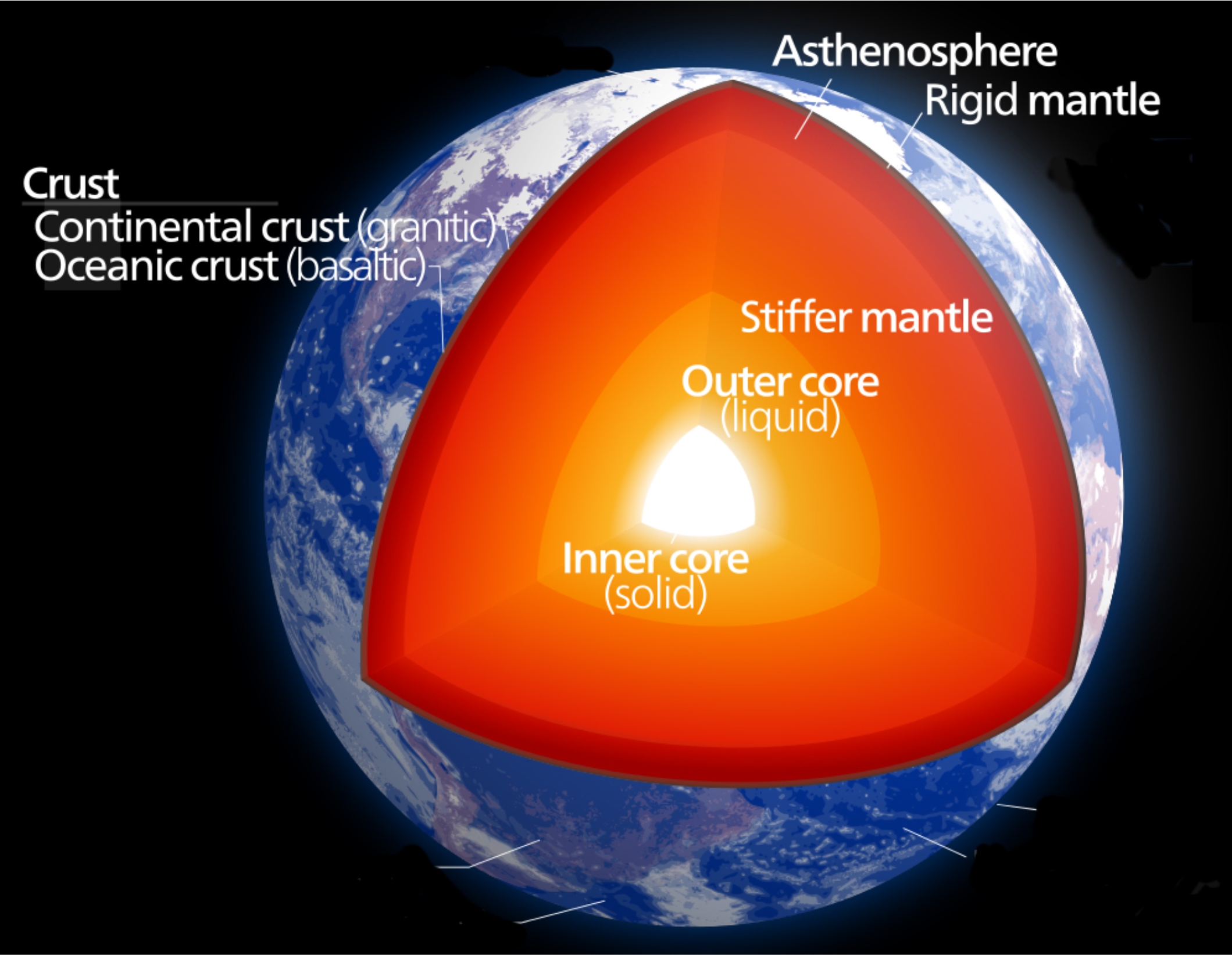
From seismic and other data, we know that the only part of Earth that is completely melted is the outer core nearly 3,000 kilometers beneath Earth’s surface (Figure 3.16). The core, however, cannot be the source of magmas that reach the outer parts of Earth. For one thing, the composition of the core is wrong. It is mostly molten iron with lesser amounts of nickel and oxygen, not silica-rich material. Additionally, no known mechanism would allow magma to traverse the 3,000 kilometers of mantle between the core and the crust. So, rocks in other parts of Earth must melt to produce the magmas responsible for igneous activity on or near Earth’s surface.
Most magmas in oceanic regions are mafic – they are basaltic. Thus, the oceanic crust consists of basalt. If the crust melted completely it could produce magmas like those erupting at mid-ocean ridges today. However, there is no evidence of complete melting of ocean crust taking place anywhere on Earth. If only partial melting occurs, laboratory and geochemical studies have shown that the melt would be much more SiO2-rich than the basalt that melted. Similar problems arise when we consider magmas in continental areas. There we find rocks formed from both mafic and silicic magmas, and geochemical studies tell us that we cannot get mafic magmas by melting continental crust. For these and other reasons, petrologists conclude that most magmas come from the (oceanic or continental) mantle. Exceptions include some silicic magmas in continental regions.
Seismic studies allow us to see where molten material accumulates in Earth, because seismic waves pass through melted, and partially melted, Earth more slowly than through solid Earth. The seismic evidence, and other evidence, reveal that magmas can be found at shallow depths in both oceanic and continental regions. In many places, magmas that reach the surface, or get near to the surface, come from 1-10 kilometers depth. However, some magmas exist and flow at deeper levels. Beneath Hawaii, for example, magma movement causes tremors as deep as 60 km. In subduction zones, seismic evidence suggests magma movement all the way down to the upper mantle.
3.3.2 The Lithosphere and the Asthenosphere
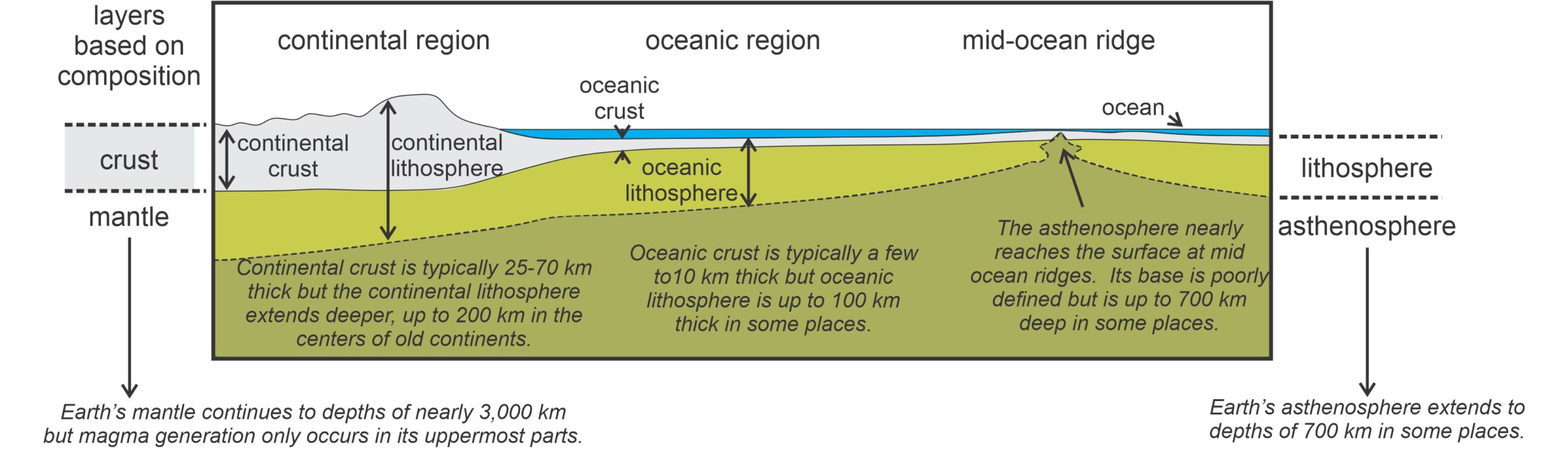
Earth’s crust and mantle have distinctly different compositions. The mantle is ultramafic overall, but the crust has a much more silicic composition. The crust and the uppermost portion of the mantle comprise the brittle lithosphere, the relatively rigid layer of Earth that forms the moving tectonic plates (Figure 3.17). Just about everywhere, the continental crust and lithosphere are thicker than the oceanic crust and lithosphere. The thickest crust and lithosphere occur beneath the centers of old continents, and the thinnest at mid-ocean ridges. Petrologists have established that the original source of most magma that reaches the surface is in the asthenosphere, which is the layer that underlies the lithosphere.
The asthenosphere is mostly solid but is partially melted in some places, notably beneath mid-ocean ridges where the lithosphere is very thin and the asthenosphere is only a few kilometers below the ocean floor. In other parts of the oceans, the asthenosphere may be beneath 100 kilometers of lithosphere, and in continental regions it is generally deeper, often as deep as 200 kilometers. Earthquake waves pass through the asthenosphere more slowly than through the lithosphere, in large part due to the presence of some melt, and geophysicists have sometimes called the upper part of the asthenosphere the low-velocity zone. Because the asthenosphere is partially melted in some places, and at or near its melting temperature in other places, it is less rigid than the lithosphere. In fact, the mostly solid material of the asthenosphere acts in some ways like a liquid; it convects (flows) at rates as fast as centimeters per year. As the asthenosphere moves, it carries the more rigid lithosphere above it, in part providing the mechanism for plate tectonics.
3.3.3 Why Melting Occurs
Just as ice melts when temperature goes above 0 oC, rocks will melt if heated to temperatures above their melting temperatures. To accomplish this rock melting requires extra heat, and that poses a problem. Where is the extra heat to come from? Although radioactive decay of potassium, uranium, thorium, or other radioactive elements may create small amounts of heat, most of Earth’s heat is left over from the original time of formation. This residual heat flows from Earth’s interior to dissipate at the surface, and Earth has been cooling for more than 4.5 billion years. In some places, flowing magma delivers extra heat, but the origin of the heat necessary to initially create the magma is problematic.
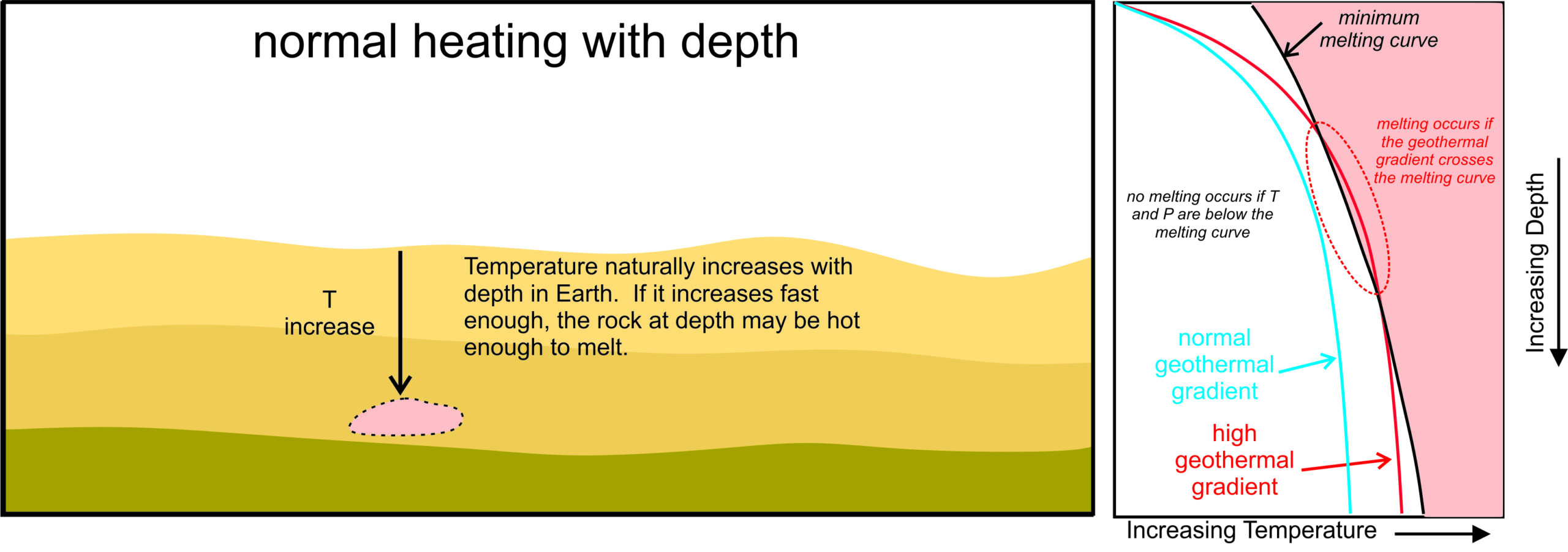
Earth’s average geothermal gradient (the rate at which temperature increases with depth in Earth) is about 25 oC/km near the surface. It is not the same everywhere – some places, such as mid-ocean ridges or hot spots like Yellowstone, have very high gradients, sometimes exceeding 50 oC/km. Other places, such as centers of old continents, have low gradients. The blue and red lines in the temperature-depth diagram on the right of Figure 3.18 are geotherms. The geotherms show schematically how temperature increases with depth for a place with an average gradient and for a place with a high gradient.
The solid black curve in the temperature-depth diagram of Figure 3.18 is a typical melting curve; it shows the minimum temperatures at which melting can occur. If temperature-depth conditions plot in the red part of the diagram, rocks will melt. The minimum melting temperature increases with depth but so do temperatures along the geotherms. In principle, if the geothermal gradient is high enough, the temperature may exceed the melting curve (shown where the red “high” geothermal gradient line crosses the black melting curve in Figure 3.18). Yet, even at mid-ocean ridges or hot spots, the gradient is generally insufficient for this to happen and cause melting. Consequently, most of Earth’s mantle is unmelted.
3.3.3.1 Melting Caused by Mountain Building
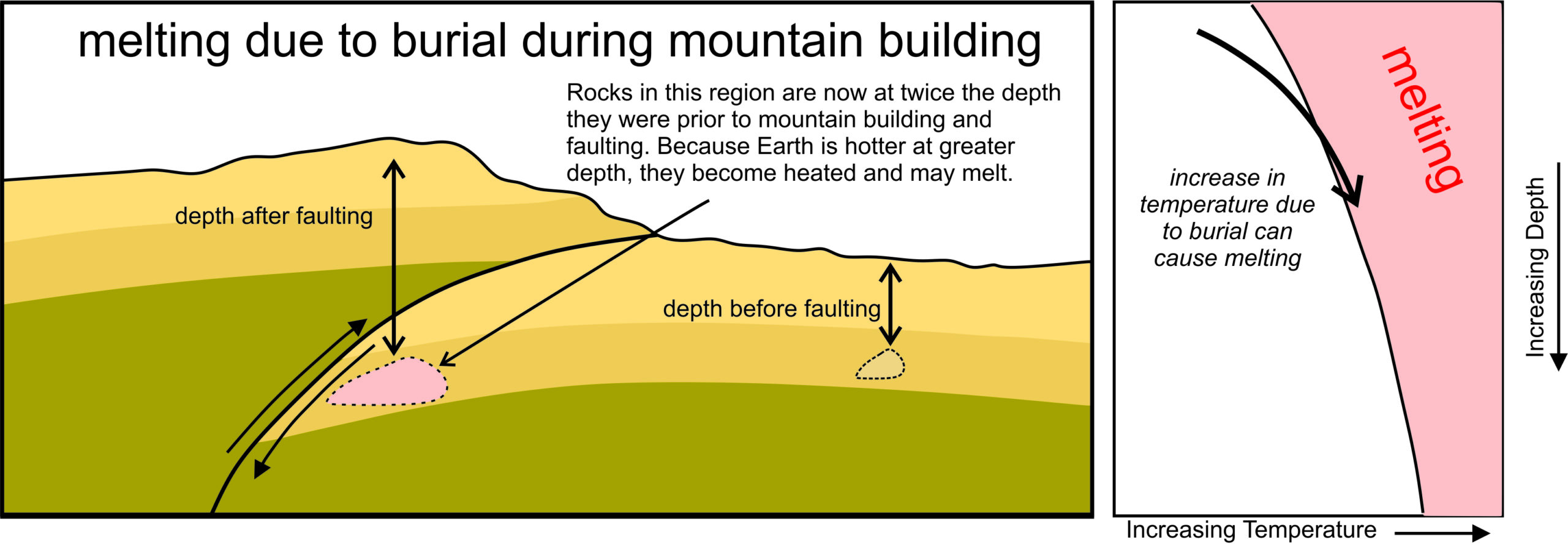
Because a normal geothermal gradient cannot lead to melting, other mechanisms must be responsible in most cases. For instance, tectonism associated with mountain building can occasionally cause melting when rocks are buried by folding or faulting – because heating naturally accompanies burial (Figure 3.19). The pressure-temperature diagram on the right side of this figure shows that if burial-induced heating is great enough, melting may occur when temperatures cross into the melting field (shown in pink). Some granites in continental regions undoubtedly form by melting of sediments and sedimentary rocks, once at or near the surface, that melted after being carried to depth.
3.3.3.2 Melting Caused by Intruding Magma
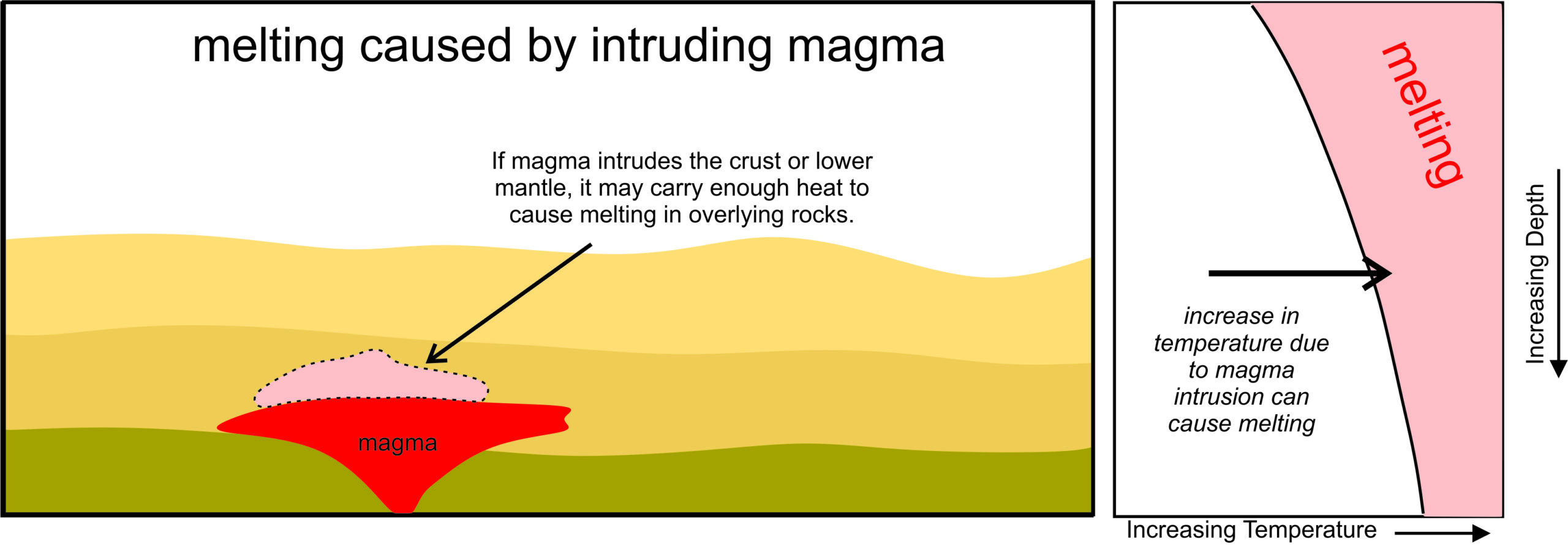
Intruding magma is a very efficient mechanism for delivering heat that can cause melting (Figure 3.20). As shown by the black arrow in the pressure-temperature diagram, heat from magmas can cause temperature to increase without any increase in pressure. As discussed earlier in this chapter, beneath Yellowstone National Park, rising magmas from the mantle are hot enough to cause overlying crustal rocks to melt. In subduction zones, magmas rising above a subducting plate may cause melting in the overlying continental lithosphere, creating silicic magmas that erupt in subduction zone volcanoes or crystallize underground to become plutons.
3.3.3.3 Decompression Melting
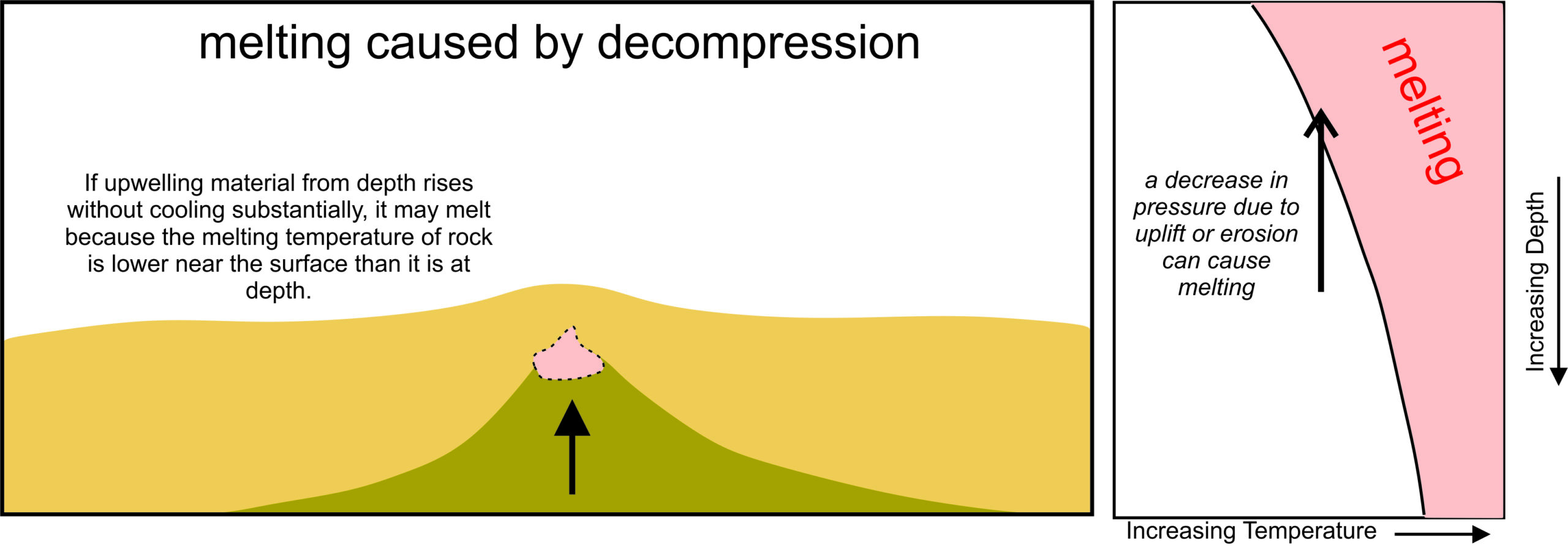
The processes described above may all cause melting, but they do not account for the widespread melting that occurs at mid-ocean ridges. There, an additional, and very significant, mechanism promotes melting – a decrease in pressure (Figure 3.21). Rising mantle moves up to fill the void created by sea floor spreading. As shown by the black arrow in the pressure-temperature diagram, the resulting pressure decrease leads to melting because rock melts at lower temperature when at low pressure, compared with high pressure. This process, called decompression melting, generates more magma than any other Earth process.
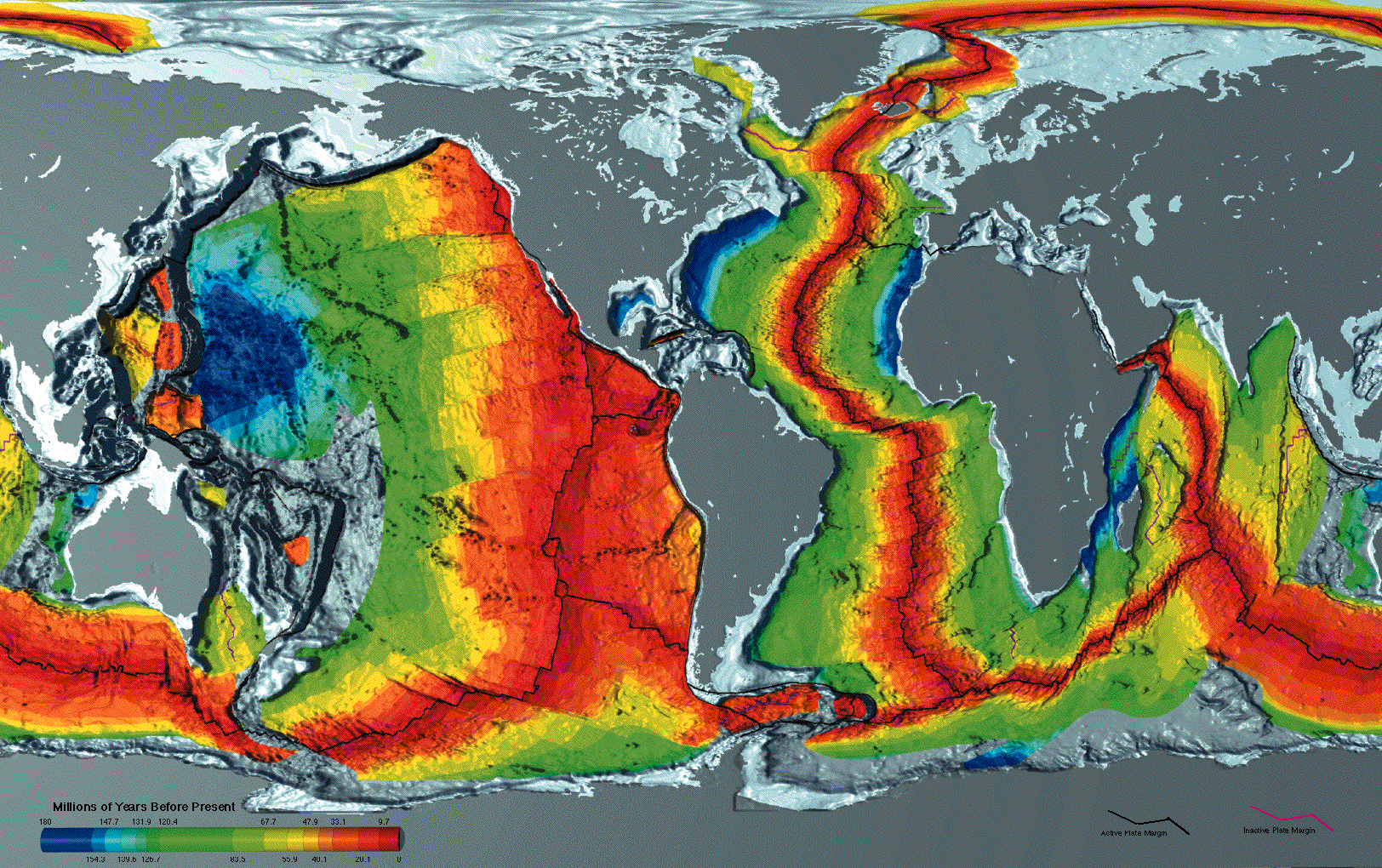
So, decompression melting is the key mechanism producing magmas at mid-ocean ridges, which, although we don’t generally see them, are the most active volcanic settings on Earth. In these settings, partial melting of rising solid rocks produces basaltic lavas that erupt on the ocean floors, and upon cooling, are added to the spreading oceanic lithosphere. Thus the youngest oceanic crust – shown in red in Figure 3.22 – is next to mid-ocean ridges and the oldest oceanic crust (light and dark blue) is found along ocean basin margins.
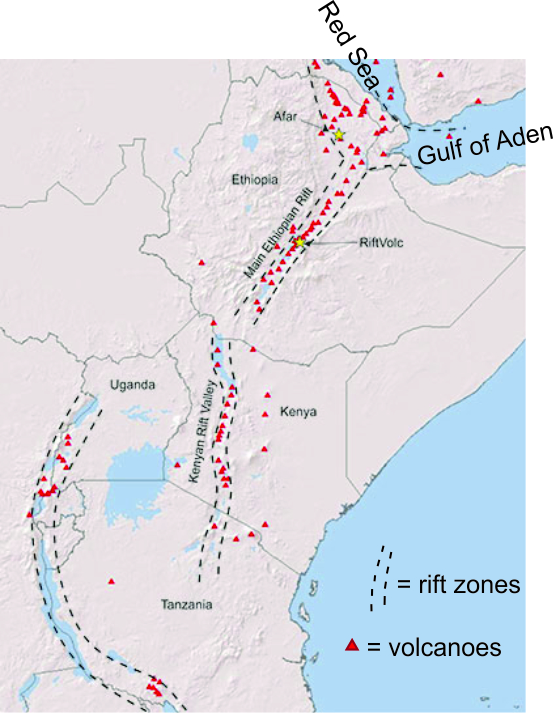
Decompression melting also leads to igneous activity where continental rifting occurs, for example along the East African Rift. The East African Rift is an elongate zone that includes the Kenyan Rift and the Main Ethiopian Rift (Figure 3.23). Along this narrow zone, the African continent has begun to split apart; the rift may eventually be the site of a new ocean basin similar to the Red Sea or the Gulf of Aden, also shown in Figure 3.23. Figure 2.13 showed some of the extensive basalt created by decompression melting in the East African Rift. Besides causing melting at rift zones, decompression contributes to the melting associated with more localized hot spots, like those under Yellowstone, where warm rocks move upwards due to buoyancy.
3.3.3.4 Flux Melting
Principles of thermodynamics tell us that two things combined will often melt at a lower temperature than they would individually. An analogy is a mix of ice and salt, which we all know melts at a lower temperature than ice alone. Similarly, rocks will melt at lower temperatures when they contain water, CO2, or another volatile compared to when dry.
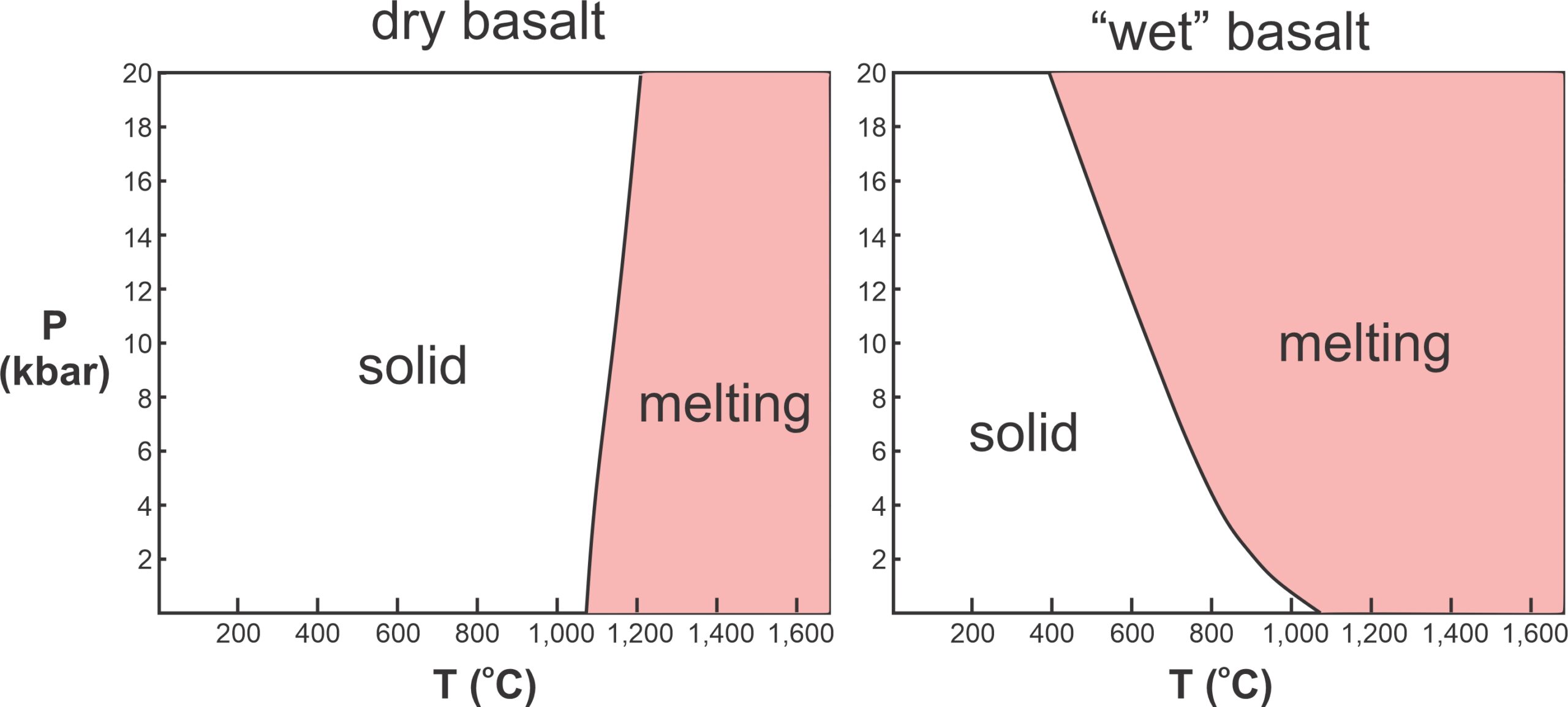
The presence of a small amount of water can change magma melting temperatures by hundreds of degrees. Figure 3.24 compares the conditions that cause melting (shown in red) of a basalt that contains no water and one saturated with a small amount of water. “Dry” basalt begins to melt at temperatures in excess of 1,000 oC. Depending on pressure, melting of “wet” basalt may begin at temperatures significantly lower. Because water and other volatiles lower melting temperatures in the same way that a flux is used to lower the melting temperature of metals, this additional mechanism for melting is called flux melting. If a rock is already hot, addition of only a small amount of water can promote melting. Water is the most important geological flux, but CO2 and other gases also promote melting in some settings.
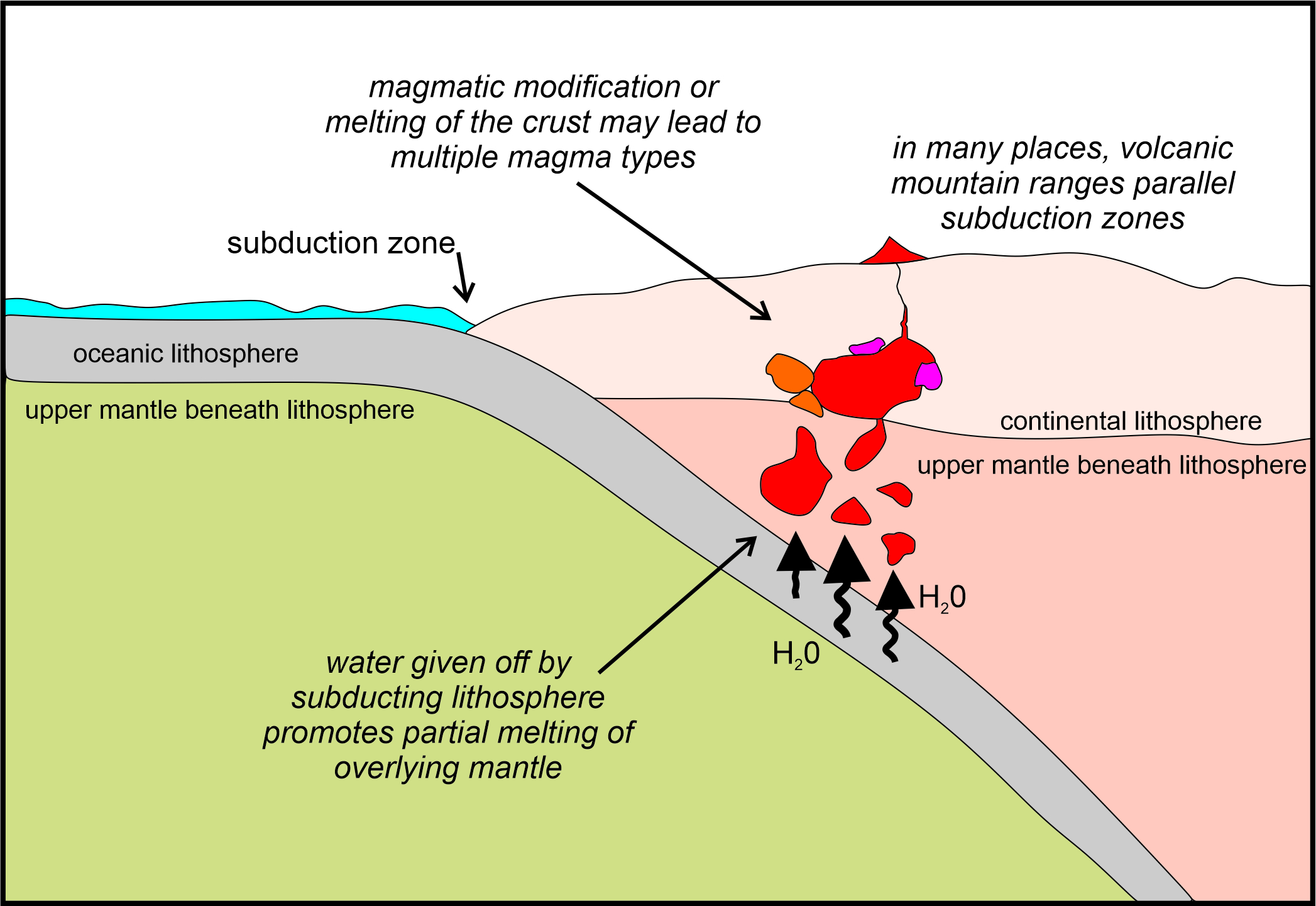
Flux melting is especially important in subduction zones (Figure 3.25). Subducting oceanic lithosphere contains hydrous minerals that react during metamorphism to form anhydrous minerals. Consequently, water is released and migrates upwards into hot overlying mantle. This water lowers the melting temperature, causing partial melting of the ultramafic mantle to produce mafic (basaltic) magma. This magma then migrates upwards and may reach Earth’s surface. It may also promote additional melting in the uppermost mantle or crust, or may become modified to produce magmas of different compositions.
3.4 Magma Movement
3.4.1 Buoyancy

Molten rock is less dense than solid rock, so molten rock is buoyant and naturally migrates upwards in much the same way that blobs of melt circulate in a lava lamp (Figure 3.26). Lava lamps contain two liquids of similar density that do not mix, a little like oil and water. Heating in the base of the lamp causes one of the liquids to expand and become less dense. Buoyancy causes the blobs to migrate upwards. As they cool, they contract, regain density, and sink back to the bottom to be heated and recycled again.
Even if only a small fraction of rock melts, the melt wants to migrate upwards. Impermeability and strength of overlying rock can resist magma movement up to a point, but laboratory experiments suggest that if only 10% of a rock melts, the buoyancy forces are great enough to cause vertical flow. The flow may form diapirs, regions where mobile, less rigid, material bulldozes its way through overlying rock. (Although the terms sound similar, diapiric flow is different from the explosive eruptions responsible for bringing diamonds to Earth’s surface. Those eruptions, called diatremes, are driven by gas pressure, not by magma buoyancy.)

Rising magma may create cracks that act as conduits in overlying rocks. This process, fracture propagation, is very important at mid-ocean ridges and produces small earthquakes, allowing seismologists to map where the fractures form and magma flows. Magma movement is easier and faster, however, if aided by fractures already in place. Sometimes these fractures reach the surface. Figure 3.27 shows lava erupting through a fracture in the eastern rift zone of Kilauea Volcano, Hawaii, in May 2018. The fracture provided a conduit for rapidly rising magma, and the rising magma caused the fracture to grow in length, leading to a classic Hawaiian fissure eruption. Similar fractures, at greater depth in Earth, act as conduits that carry magma toward the surface.
As magma moves upwards, it cools and crystallizes, two processes that lead to an increase in magma density, which slows upward flow. In fact, movement will stall if the magma reaches a level in Earth where surrounding solid material has the same density as the magma does, so the magma may never reach Earth’s surface. Many factors determine where magma movement will stop, including magma composition, gas content, temperature, viscosity, and the nature and amount of crystalline material in the magma. The rate of movement is important because magmas must move relatively quickly or they will stop flowing. The temperature of the crust is also important: if the crust is warm, the magma will not cool as quickly and so will migrate more easily to upper levels. Consequently, volcanic regions, where Earth’s crust is warm, tend to sustain volcanism for extended durations.
The race to the surface determines if rising magmas will erupt or if they will crystallize at depth. In general, magmas coming from the base of the lithosphere take, at most, days to years to reach the surface, provided fracture conduits are available. For example, studies of basalts in Patagonia (Argentina and Chile) have shown that the magma ascent rate was about 5 meters/second and that it only took the mafic magma 2-8 hours to get from its source region to the surface. The Patagonian magma followed well-developed fracture systems. Other studies suggest that if the only upward movement is due to diapirism, the rate of ascent may be 1,000 to 10,000 times slower! Magmas that rise at rates faster than about 0.1 m/sec account for most explosive eruptions. Slower magmas generally account for effusive eruptions; there are, however, exceptions.
3.4.2 Magma Chambers and Cooling
Magma chambers underlie all active volcanic regions. These chambers, containing partially melted rock, are sometimes under great pressure. Geologists have drilled into magma chambers several times, including twice in Iceland and once in Hawaii. These few incidences were significant because they allowed sampling of magma from in-place instead of after an eruption.
Magma chambers may exist for millions of years but are not static. Over their lifetimes, they inflate incrementally when fed by new magma and deflate during times of igneous activity. Beneath Hawaii, the chambers are at depths of 3-4 kilometers, beneath Japan 8-10 kilometers, beneath Alaska’s Aleutian Island 7-17 kilometers, and beneath Iceland 20 kilometers. In continental regions, the chambers may be deeper. The chambers range from very small to very large, and some can produce immense eruptions. The 1815 eruption of Mt. Tambora, in Indonesia, ejected more than a trillion cubic meters (1,000 cubic kilometers) of material – mostly ash and other debris thrown into the air. Prehistoric eruptions in Yellowstone, at Long Valley (California), and at Toba (Indonesia) were much larger.
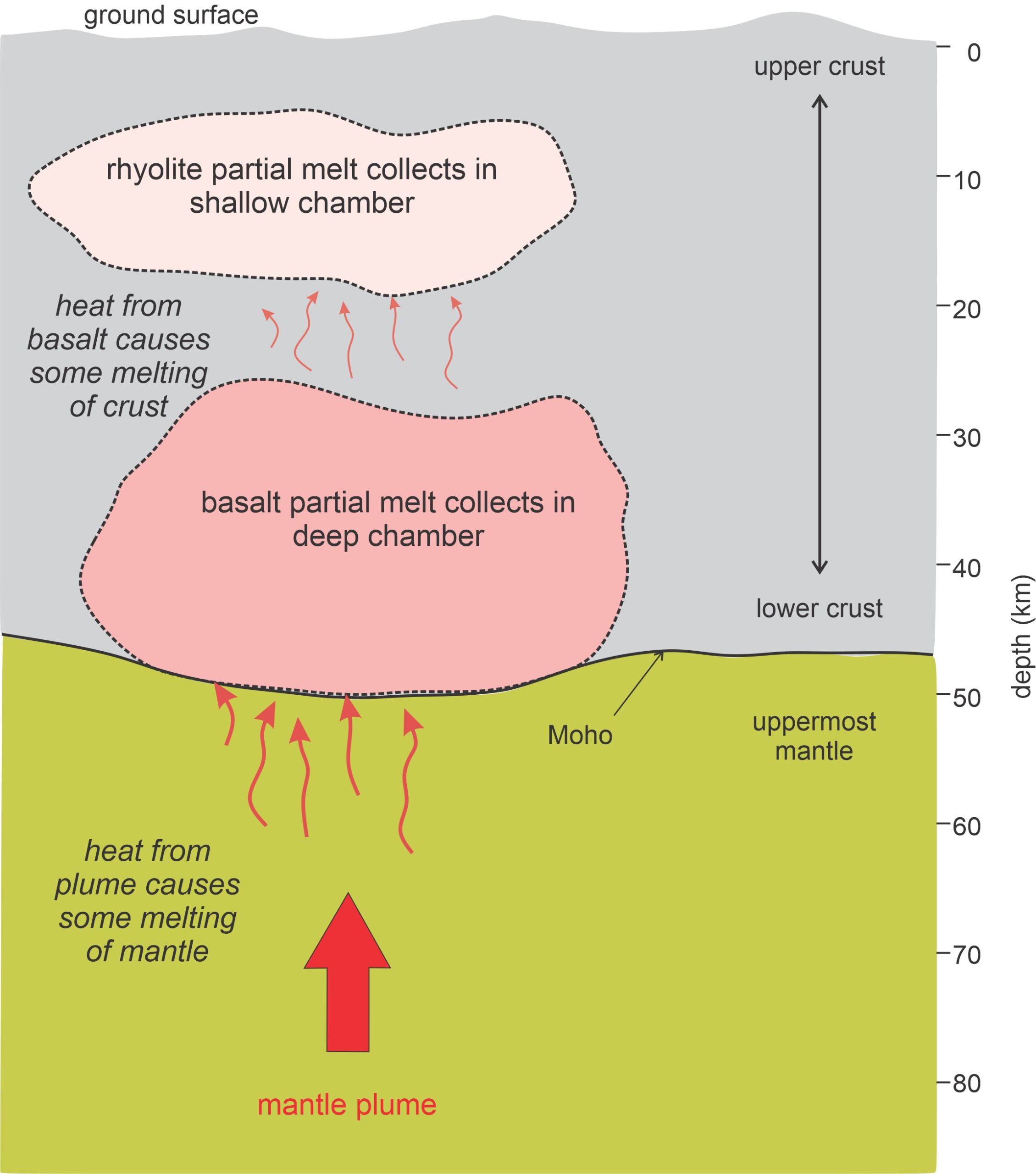
Magma chamber plumbing and geometry may be complex. Seismologists studying the Yellowstone region have found two magma chambers beneath Yellowstone National Park (Figure 3.28). An upper silicic (rhyolitic) chamber is found at 10-20 kilometers depth, and a deeper mafic (basaltic) chamber is just above the base of the crust (Moho) at 30-50 kilometers depth. These chambers, like most places where magma exists, are only partly melted. Seismic wave velocities suggest the upper chamber is about 30% melted. The lower chamber may only contain a few % melt. See also Figure 3.4, earlier in this chapter.
3.4.3 Cumulates

Because magma chambers contain a mix of crystals and molten material, gravity may cause them to become differentiated. Dense crystals sink, so, given enough time, the chambers may become layered as crystal cumulates collect in their bottoms. Figure 3.29 shows black layers of chromite that accumulated in layers due to gravity. The cumulates are surrounded by anorthosite, a kind of rock composed almost entirely of plagioclase.
Some mineral crystals will float on top of denser magmas, which also can lead to layers of different compositions. Thus, a single homogeneous chamber may become separated into zones of different compositions and produce magmas of more than one composition during subsequent eruptions. For instance, the 79 AD eruption of Mt. Vesuvius near Naples, Italy, produced bimodal volcanism thought to represent magmas from different levels in the underlying magma chamber.
3.5 Melting of Minerals and Rocks
3.5.1 Incongruent Melting

Different minerals melt at different temperatures. Ice, for example, melts when the temperature reaches 0 oC at one atmosphere pressure, but gold (shown melting in Figure 3.30) melts at 1,064 oC. Many minerals melt at temperatures above the melting temperatures for ice and gold. Quartz, for example melts at about 1,700 oC at one atmosphere pressure, and Mg-olivine (forsterite) melts at 1,890 oC. When ice, gold, quartz, or forsterite melt, the composition of the melt is the same as the solid. Thus, ice melts to produce water, molten gold has the same composition as solid gold, molten quartz is SiO2, and molten forsterite is Mg2SiO4.
Ice, halite, quartz, and forsterite melt at specific temperatures (that vary if pressure varies), and the composition of the liquid that forms is the same as the composition of the solid. In contrast, K-feldspar, plagioclase, and many other minerals, melt incrementally over a range of temperatures at a given pressure. When they melt, the composition of the melt is different from the original solid until all is melted. Minerals that melt at a single temperature are said to melt congruently; those that melt over a range of temperatures melt incongruently.
Many minerals are solid solutions, meaning that their chemical compositions are not fixed; compositions vary within limits. Solid solution minerals melt over a range of temperatures, and melting and crystallization temperatures depend on mineral composition. While melting, melt and crystals of different compositions coexist. Plagioclase, the most common mineral in Earth’s crust, is a good example. Plagioclase may have any composition between anorthite (CaAl2Si2O8) and albite (NaAlSi3O8) – NaSi and CaAl may substitute for each other. When a plagioclase of intermediate composition begins to melt, it melts incongruently. The first melt is more albite-rich than the original mineral. Consequently, after partial melting occurs, the solid plagioclase that remains must be more anorthite-rich than the original mineral.
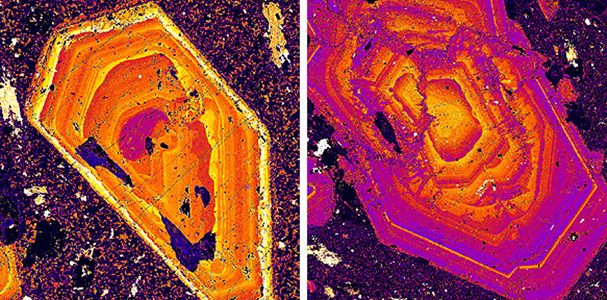
Crystallization of molten plagioclase is just the opposite of melting. The first crystals to form will be more anorthite-rich than the magma. As temperature drops, more, and larger, crystals will grow until all the melt is gone. As this occurs, the composition of the crystals will change, becoming more albite-rich. When crystallization is complete, the final crystal will have the same composition as the original melt. If equilibrium conditions are maintained, as crystallization proceeds, plagioclase crystals should be homogeneous – they should have the same composition throughout. In laboratory experiments, this may happen, but in nature it sometimes does not. The two images in Figure 3.31 show compositional zoning in two plagioclase feldspars from an igneous rock. The images were obtained using a scanning electron microscope, and the colors show the distribution of calcium and sodium (purple zones are more sodium rich). If the crystals had stayed in equilibrium with the melt as they grew, the compositions would be uniform and no concentric rings would be visible. However, the rings, showing compositional variation, are typical for many plutonic rocks.
We will look at incongruent melting in more detail later when we look at phase diagrams. For now it is sufficient to recognize that partial melting of minerals can produce melts that are not the same composition as their parent material. And partial melting of rocks just about always does the same. This is another way that rocks and magmas may become differentiated.
3.5.2 Liquidus and Solidus Temperatures
Because rocks generally contain multiple minerals, most that melt incongruently, rocks melt over a range of temperatures. When a rock or mineral is heated, the temperature at which melting begins is termed the solidus temperature (because all is solid below that temperature). The temperature above which all is liquid is the liquidus temperature. At temperatures between the solidus and liquidus, solid material (having composition different from the original solid material) will coexist with melt of a different composition. For materials that melt congruently, the solidus and liquidus temperatures are the same as the melting point. For those that melt incongruently, solidus and liquidus temperatures may differ by hundreds of degrees.
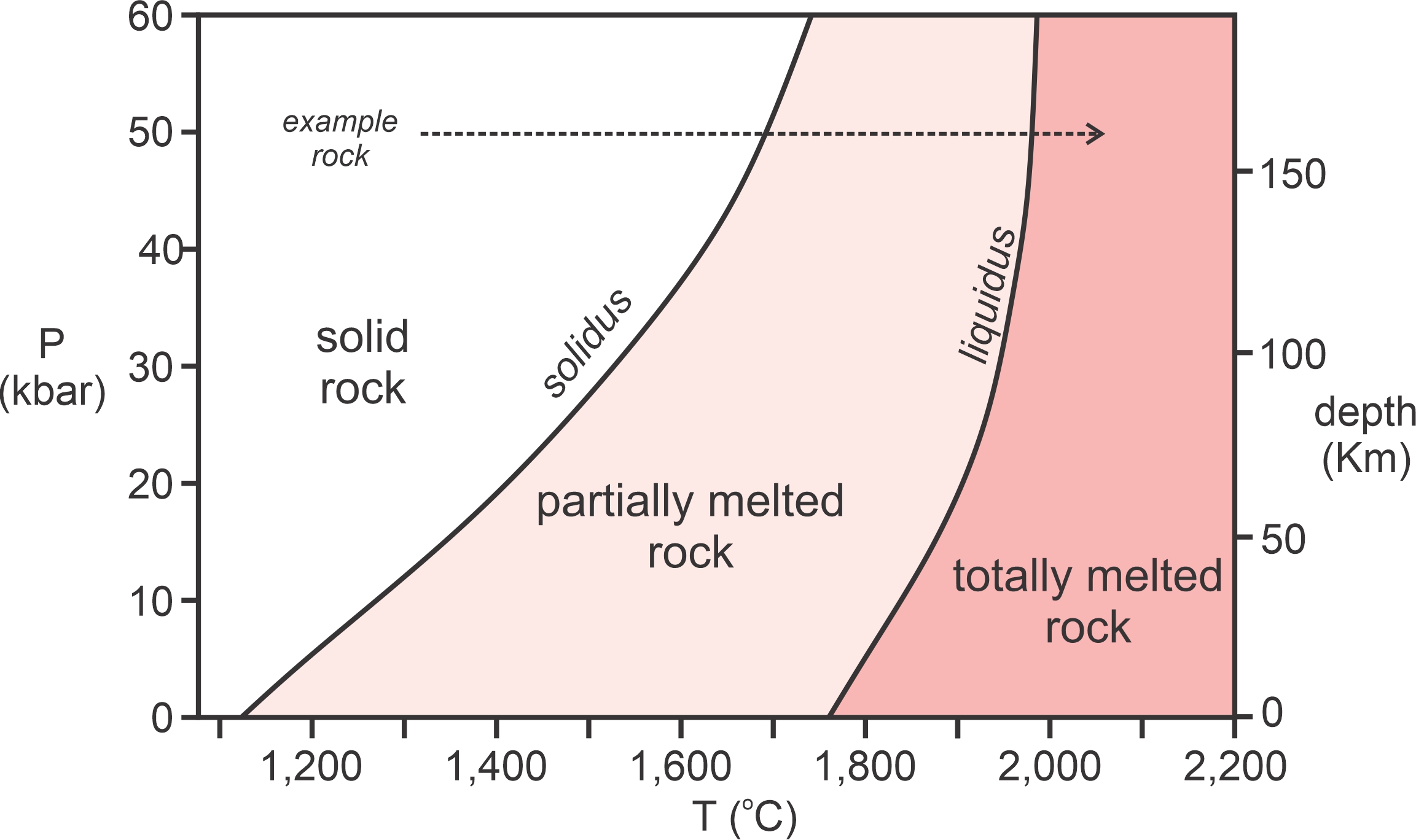
Figure 3.32 is a schematic diagram showing the liquidus and solidus for ultramafic rock, the kind of rock that dominates Earth’s mantle. Similar diagrams exist for rocks of other compositions. The diagram is a pressure-temperature graph showing liquidus and solidus temperatures for different pressures. Pressure increases with depth in Earth, and the scale on the right shows increasing depth with pressure.
Consider, for an example, solid rock at 50 kbar pressure (160 kilometers depth). If it is heated (dashed line, Figure 3.32), it will begin to melt at around 1,700 oC. Continued heating will cause more melting until temperature reaches about 1,970 oC. Then, all will be melted and the melt will have the same composition as the original rock. At temperatures below the solidus, all is solid, at temperatures between the solidus and liquidus, partial melting occurs, and at temperatures above the liquidus all is melted.
As shown in Figure 3.32, solidus and liquidus temperatures are not the same at all pressures – melting normally occurs at higher temperature with increasing pressure. Consequently, the liquidus and solidus temperatures for rocks are greater at greater depths in Earth. As previously pointed out, temperature increases with depth, too. If it increases enough, it may intersect the mantle solidus, leading to partial melting. However, complete melting of the mantle would require temperatures to exceed the liquidus, and such conditions do not exist within Earth. Most studies have concluded that the amount of partial melting at depth in Earth rarely exceeds just a few percent.
The notion of partial melting is already complicated, but adding more complication, the products of melting may be different at different pressures. For example, at low pressure (Earth’s surface or shallow depths), enstatite (Mg2Si2O6) melts incongruently to produce forsterite (Mg2SiO4) and a melt that is more silica-rich than enstatite. At high pressure (deep within Earth), enstatite melts congruently. In contrast, the pressure effect on forsterite melting goes the other way. Forsterite melts congruently at 1 atmosphere but melts incongruently, at very high pressure, to produce periclase (MgO) and a liquid more silica-rich than forsterite is (Table 3.2).
| Table 3.2 Melting Reactions for Enstatite and Forsterite | |
| pressure | melting reactions |
| low | enstatite = forsterite + melt (incongruent) forsterite = melt (congruent) |
| high | enstatite = melt (congruent) forsterite = periclase + melt (incongruent) |
3.5.3 Bowen’s Reaction Series
When rocks melt or magmas crystallize, things are generally more complicated than when a single mineral melts or crystallizes. Most rocks contain more than one mineral, and different minerals melt or crystallize at different temperatures. N. L. Bowen, an early 20th century petrologist, conducted many laboratory experiments and was the first to compare melting and crystallization temperatures of common igneous minerals in rocks.
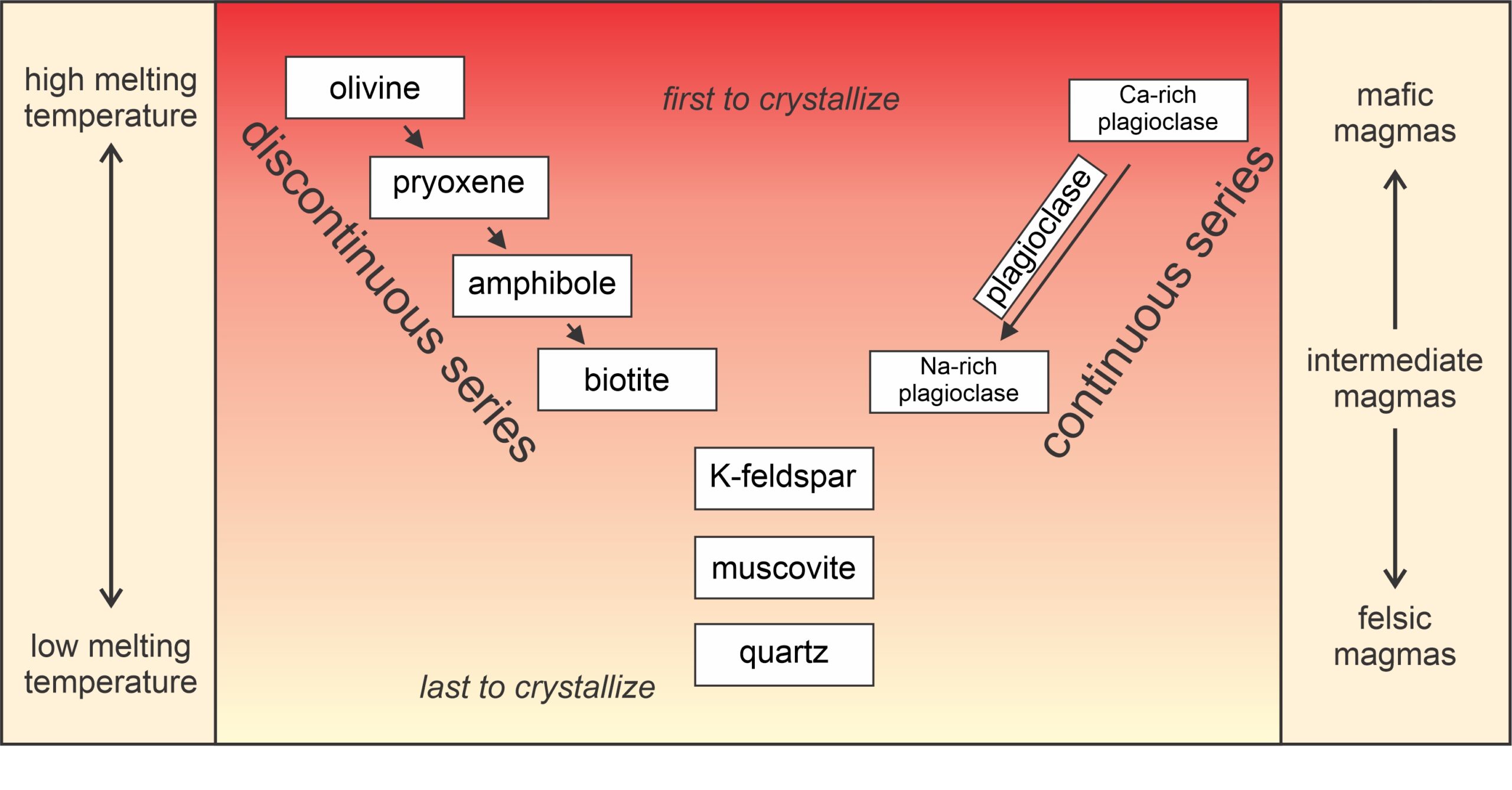
Bowen’s Reaction Series (Figure 3.33) depicts Bowen’s fundamental findings. We call it a reaction series because during melting (or crystallization) solid minerals continuously react with surrounding liquid.
Mafic magmas crystallize at high temperatures and felsic magmas at lower temperatures. Bowen’s series shows the relative liquidus temperatures for common minerals for magmas of different compositions. As shown by the series, olivine crystallizes at the highest temperature (from mafic magmas) and quartz at the lowest temperature (from felsic magmas).
One sometimes confusing thing about this series is that it depicts liquidus temperatures for minerals when they crystallize from magmas of complex chemistry. Crystallization temperatures are different for the individual minerals if they are by themselves. For example, ignoring minor complications involving polymorphs, quartz may not begin to crystallize from a granitic melt until other minerals have formed and temperature drops to less than 1,275 oC. In contrast, quartz will crystallize from a melt of 100% SiO2 composition at a much higher temperature (in excess of 1,700 oC).
Plagioclase melts incongruently over a range of temperature depending on its composition; Bowen called the plagioclase side of the diagram the continuous series. Most other minerals, also depending on their compositions, melt sequentially over more restricted temperature ranges (discontinuous series). Bowen found that (mafic) minerals common in ultramafic and mafic rocks have the highest liquidus and solidus temperatures, and (silicic) minerals that are common in silicic rocks have the lowest. Consider a cooling magma: as temperature decreases, minerals higher up in the series crystallize first followed by minerals lower down. We call minerals that melt and crystallize at high temperatures high-temperature minerals; those that melt and crystallize at low temperatures are low-temperature minerals. The specific minerals that crystallize, however, vary with magma composition.
Although melting and crystallization in the order depicted by Bowen’s Reaction Series seems straightforward, there are many complications. No magmas follow the entire series – most crystallize only one or a few of the minerals in the series – and some magmas crystallize minerals that are not part of the series. Furthermore, some minerals melt (and crystallize) congruently and some do not. Additionally, many melting and crystallization reactions involve more than one mineral reacting together. For example, at Earth’s surface, anorthite melts at about 1,560 oC and diopside melts at about 1,390 oC. A rock that contains both anorthite and diopside, however, will begin to melt at around 1,270 oC, a much lower temperature than the melting point of either individual mineral.
For most magmas, crystallization begins at some maximum temperature and continues over a range of temperatures until everything is solid. As the process continues, different minerals form at different temperatures and the composition of the magma changes. The opposite occurs during heating of a rock. Melting generally begins by melting of low-temperature minerals followed sequentially by melting of higher-temperature minerals until all is liquid. During this process, the melt continually changes composition. Bowen’s reaction series is a model that serves to remind us that the minerals that form depend on magma composition, that different minerals melt and crystallize at different temperatures, that mafic minerals tend to crystallize before silicic ones, and that silicic minerals melt at lower temperatures than mafic minerals do. The series does not, however, apply in detail to any known magma or rock composition. And, as pointed out above, the series only applies to rocks and magmas ‒ it does not tell us about the melting and crystallization temperatures of individual minerals when they are by themselves.
3.6 The Importance of Partial Melting and Fractional Crystallization
3.6.1 Incomplete Melting
Melting can only occur if temperature exceeds the solidus, and temperatures rarely, if ever, reach the liquidus. Because the geothermal gradient is different in different places, this means that partial melting occurs but does not occur everywhere. So, magmas generally form by melting of an originally solid parent rock that does not melt completely. When a rock melts only partially, producing a melt that contains melted low-temperature minerals and leaving behind solid high-temperature minerals, we call the process anatexis. In the mantle, for example, anatexis of ultramafic rock produces basalts.

Migmatites (from the Greek migma meaning mixture, and ite, referring to rock) are rocks composed of two different components that are mixed, or swirled, together. Typically migmatites contain a light colored segregation that in many cases appears to have formed by partial melting of the darker surrounding material. In the crust, many migmatites, such as the one seen in Figure 3.34, are thought to have formed by anatexis associated with metamorphism of a parental sedimentary rock. The result is a mixed rock that contains both metamorphic and igneous components. The melt that develops eventually cools and crystallizes just like any other magma does, and will sometimes contain large crystals (phenocrysts) after completely solidified. If the melt migrates away from where it was produced, identifying its origin may become problematic, and the residual material left behind will not resemble the original sedimentary parent. It seems apparent, nonetheless, that large scale anatexis of crustal rocks can produce large volumes of granitic melts that later form granitic plutons. The plutons may contain xenoliths (included unmelted pieces) of the original rock that melted to form the granitic magma.
3.6.2 Equilibrium or Not?
In an equilibrium melting process, the melt and solid remain in contact and in chemical equilibrium as melting occurs. The system is “closed” – the overall composition does not change – so the melt and remaining solid material add up to the starting composition. Consider the melting process that may occur when a rock is heated. Melting begins at the solidus temperature, and the first melt is formed by the melting of low-temperature minerals, singly or in combination. The rest of the minerals remain unmelted. Melting progresses as temperature increases, and different minerals melt at different temperatures.
As the amount of melting increases, the melt composition evolves to be more like its original parent rock until everything has melted. During this process, the minerals present will change, and the compositions of solid solution mineral crystals will change as atoms migrate in and out of the solid crystals. It does not matter if the rock melts partially or completely; if melt and solids continue to react, chemical equilibrium is possible as compositions change in response to temperature changes. The same concept of equilibrium applies to crystallization. If equilibrium is maintained during crystallization, crystals will be homogeneous in composition and will change proportions and compositions systematically as temperature decreases. However, disequilibrium can occur if the migration of atoms through the solid crystals, or through a viscous melt, is not fast enough to keep up with cooling.

When studying rocks, petrologists may find it difficult to decide if crystals and melt stayed in equilibrium. Some volcanic rocks, however, contain zoned crystals that are evidence of disequilibrium. We saw two examples in Figure 3.31. Figure 3.35 is another example. It shows a polarizing microscope view of a large compositionally zoned grain of clinopyroxene in a basalt. The dark material surrounding the clinopyroxene is mostly volcanic glass, and the needle-shaped light colored crystals are plagioclase. If the clinopyroxene grain was homogeneous, the colors induced by the polarizers would be the same in all parts of the grain. Zoning of this sort is evidence that the melt and crystals did not stay in equilibrium during crystallization. Figure 3.31 shows similar zoning in plagioclase. In both cases, the centers of the crystals grew at high temperature, and as temperature decreased the crystals grew larger. If the minerals and melt stayed in equilibrium, the grain (no matter the size) would have a homogeneous composition. But in zoned crystals the crystal cores have compositions formed at higher temperature than the rims did. The outer zones have compositions that formed at lower temperature because atoms could not migrate into and through the crystals fast enough to maintain compositional homogeneity. Thus, only partial equilibrium was maintained. Many volcanic mineral crystals have broad homogeneous centers but are zoned near their rims, suggesting that they stayed in equilibrium with the melt until the latest stages of crystallization.
3.6.3 Partial Melting
Large scale disequilibrium melting occurs if a melt and a solid do not continue to react together, but instead become chemically isolated due to physical separation. For example, if a rock melts partially and the magma escapes upwards, the melt and remaining solid material cannot react to stay in chemical equilibrium.
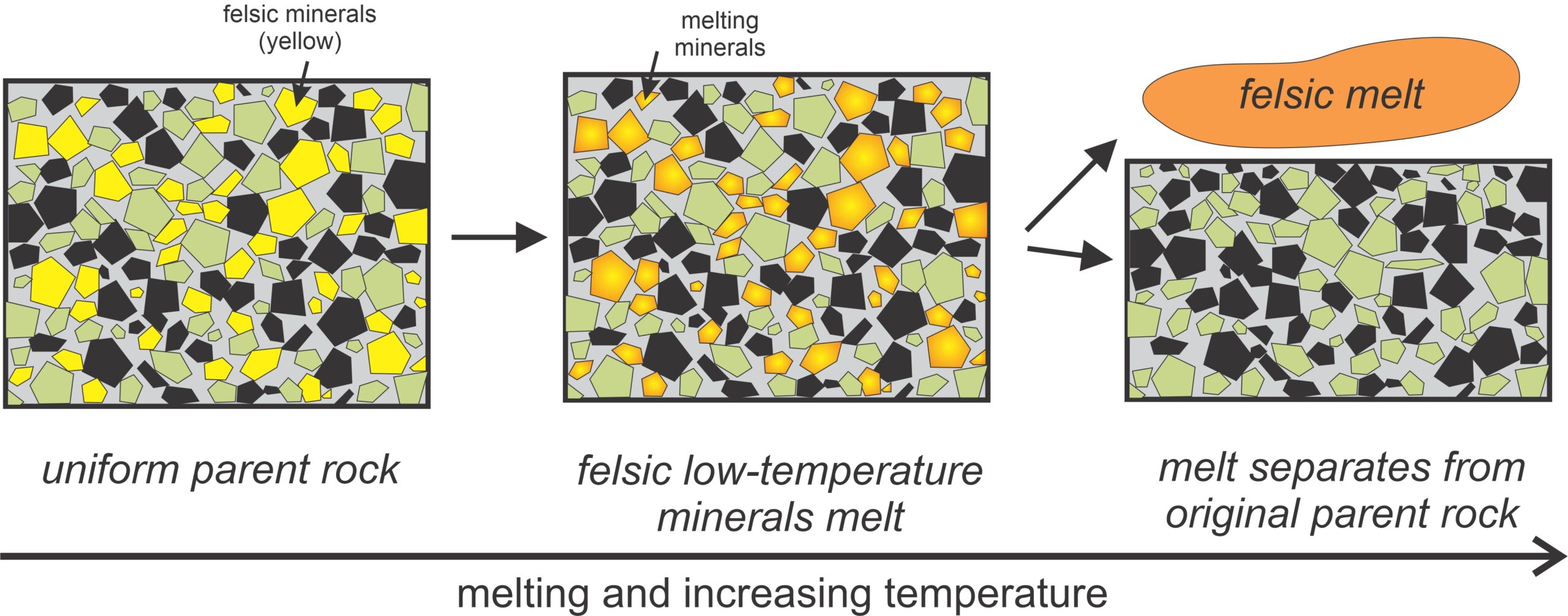
Figure 3.36 shows melting of an original parent rock that contains several different minerals.. The first minerals to melt are (generally Si-rich) low-temperature minerals (shown in yellow). So, initial melting produces a relatively silicic magma (shown in orange). This melt may subsequently become separated from the leftovers of the original rock. Consequently, a melt of different composition from the parent has been produced and may move upwards in Earth.
Partial melting is a widespread and important process that occurs in the source region for most magmas. Low-temperature minerals always melt first, either individually or in consort with others. They are relatively silica-rich minerals compared with others in a rock, so when partial melting occurs, the melts are more silicic than the parent rock is. The remaining rock becomes depleted in silicic components and, therefore, more mafic than its parent. When this happens, silicic melts migrate upwards, leaving more mafic residue behind. So, partial melting explains, in part, why the Earth has differentiated into a more silicic crust and more mafic mantle during its 4.6 billion year lifetime. These generalizations always apply, but the specific products of partial melting depend on the starting material composition and the amount of melting. Furthermore, the results will be different if the parent rock is already depleted.
Earth’s mantle is ultramafic; if it melted completely it would produce ultramafic magma, but, as discussed previously, complete melting cannot occur because there is no known mechanism for heating the mantle to the very high temperatures needed to melt it completely. Partial melting, however, is common, and the upper mantle is the source of many magmas that move within the crust and sometimes reach the surface. Because the upper mantle has a relatively uniform composition, partial melting of mantle produces similar magmas worldwide, almost all mafic, equivalent to basalt compositions. More silicic magmas may also be generated in the mantle, but they are uncommon. Similarly, partial melting of subducted ocean crust, which is basaltic everywhere, generally produces magmas of intermediate composition, and partial melting of lower continental crust produces silicic magmas (equivalent to granite).
3.6.4 Fractional Crystallization
Fractional crystallization, the opposite of partial melting, occurs when a magma partially crystallizes and the remaining magma becomes segregated from the crystals. In these circumstances, the new evolved magma will have a different composition from its parental magma. The evolved magma, which is more silicic than its parent was, may move upwards, leaving the high-temperature (mafic) minerals behind. Fractional crystallization, like partial melting, has been a key process contributing to differentiation of Earth.
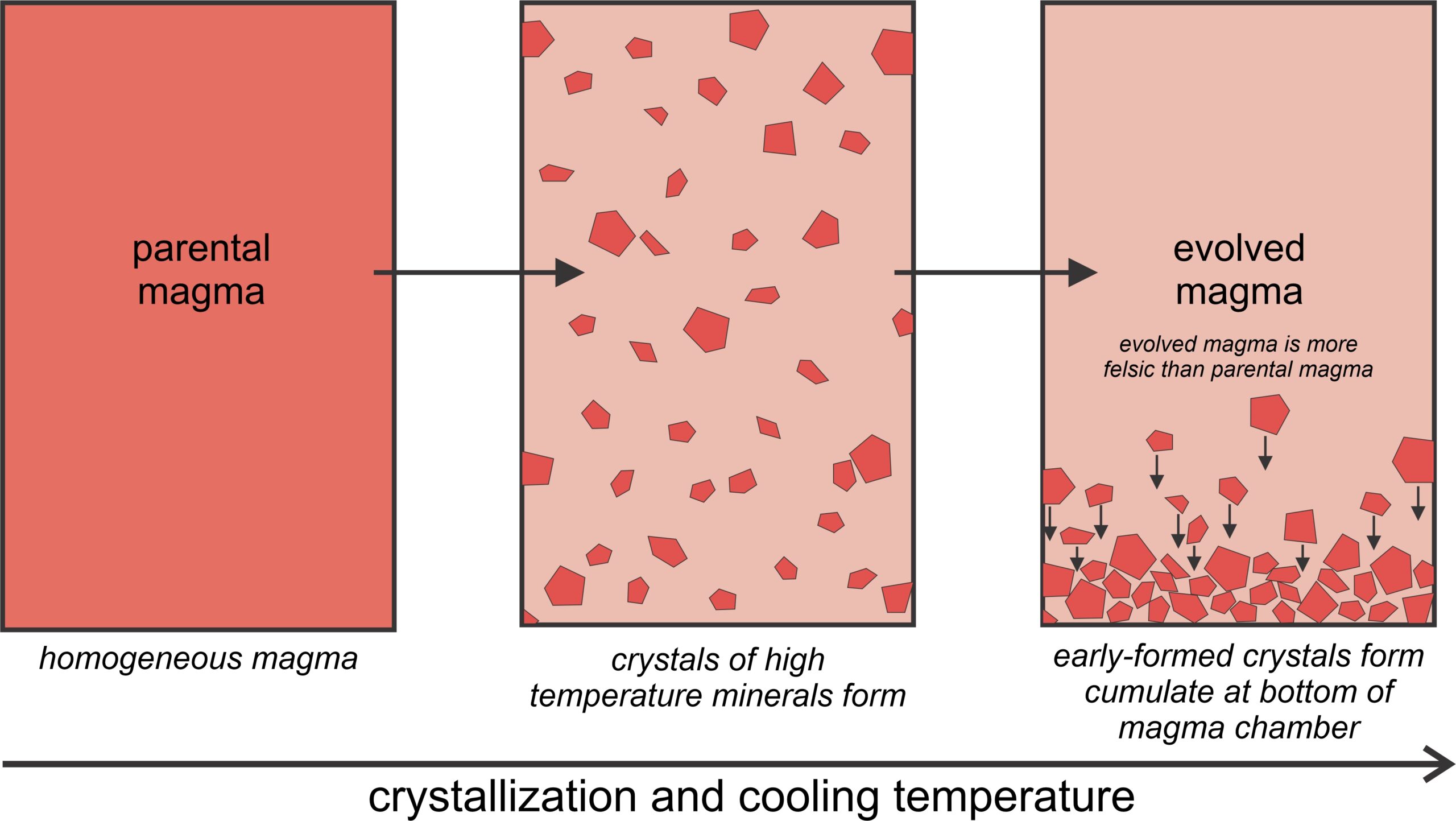
Fractional crystallization may occur when newly formed crystals sink to the bottom of a magma chamber and no longer stay in equilibrium with the melt. Figure 3.37, a schematic diagram, shows the principles involved. While cooling, a parental magma crystallizes some high temperature minerals. These minerals eventually sink to the bottom of the magma chamber, leaving an evolved magma above. Because high-temperature minerals are mafic, the evolved melt is more silicic (less mafic) than the original parent magma. During this process, a cumulate rock forms at the bottom of the magma chamber, and the evolved magma may move upwards and become completely separated from the cumulate. Fractional crystallization explains the origins of cumulate rocks like the chromite cumulates shown in Figure 3.29.
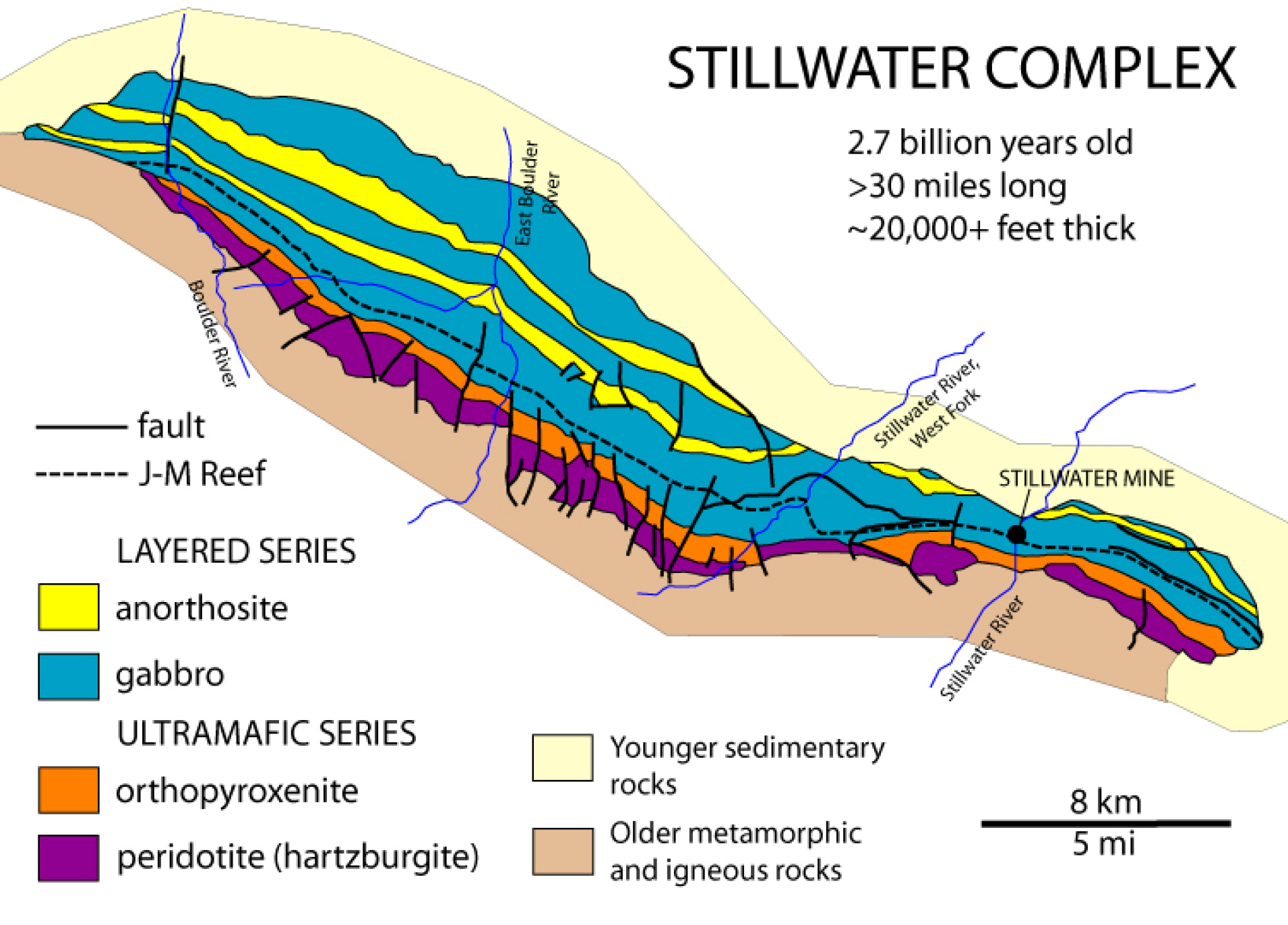
The Stillwater Complex near Nye, Montana, provides a spectacular and complicated example of fractional crystallization (Figure 3.38). The complex contains a layered series of ultramafic and mafic rocks emplaced during multiple intrusive events. All intrusions involved fractional crystallization and as each magma cooled, cumulate layers containing olivine, pyroxene, and chromite settled in the bottom of the magma chambers, leaving a less mafic magma to crystallize in the upper parts of the chambers. So, the complex is layered, with more mafic rocks in the lower parts of each intrusive body. The layers formed horizontally but tectonism tilted them, making it easier to see all the different rocks in outcrops today. To geologists, Stillwater is famous for the spectacular rocks that are exposed, and for the fascinating information revealed about fractional crystallization. Economically, Stillwater is famous because it hosts one of the two platinum/paladium mines in North America (Box 3.2).
3.6.5 Other Processes Explaining Variations in Magma Composition
Fractional crystallization is undoubtedly the most important process that changes magma composition after a magma forms. Other mechanisms, however, also lead to changes. For example, in some settings, hot magmas may melt surrounding rocks and assimilate them into the magma. Generally, we think of this assimilation occurring when mafic magmas encounter more silicic rocks, because mafic magmas may be hotter than the silicic rock’s melting temperature is. So, assimilation can make magma more silicic and is most likely to occur in the (silicic) crust. Some volcanic rocks contain crustal xenoliths, inclusions of rock fragments incorporated as solid pieces into the melt; often the xenoliths show evidence of partial melting. It is no stretch to assume that sometimes xenoliths melt and mix in completely. Some geochemical data, too, supports the idea that crustal material has been incorporated into a mantle-derived melt.
Different magmas may also combine to produce hybrid magmas of different compositions. However, magma mixing is unlikely to happen if magma compositions are too different because different magmas have different melting temperatures, densities, and viscosities. Although some evidence suggests that magma mixing occurs on a small scale, most petrologists believe it is generally a minor contributor to magma diversity. A third process, liquid immiscibility, has also been proposed as a process that may lead to change in magma composition. Immiscible liquids unmix in much the same way that chicken soup separates into broth and fat upon cooling. Experimental evidence suggests, for instance, that sometimes a sulfide-rich melt may unmix from mafic silicate magma – a potential important process forming ore deposits, or that alkali-rich magmas may unmix from less alkaline ones. Some petrologists have invoked this last process to explain the origin of carbonatites, the unusual carbonate-rich magmas we mentioned briefly in Section 3.2.2 of this chapter. We will look at immiscibility in more detail later when we look at phase diagrams.
3.6.6 Parental Magmas and Differentiation
Only a few rare magmas may not be evolved. For example, the white veins (termed leucosomes) in migmatites that form by partial melting of sedimentary rocks may not have changed composition after they formed (Figure 3.34). The leucosomes appear to have been created by partial melting of metasedimentary rock, and the melt has remained local and has not differentiated.
The majority of magmas, however, evolve from some parental magma. They are evolved melts, not melts having the composition created during initial melting. Subsequently, as crystallization progresses, magma compositions follow what is called a liquid line of descent, producing a series of magmas of different compositions as fractional crystallization removes specific minerals from the melt.
If solid mantle melted directly, either partially or completely, to create magma, the magma would be called a primary magma. Primary magmas have undergone no differentiation and have the same composition they started with. Specifically, if they come from the ultramafic mantle, and were not subsequently modified, they must have a very high Mg:Fe ratio and be enriched in Cr and Ni just like mantle rocks, and petrologists use these and other characteristics to test if magmas could be primary magmas. Most magmas fail the tests, and primary magmas are exceptionally rare, or may not exist at all. Some magmas and rocks, however, come close to being primary, and petrologists describe them as primitive, meaning they have undergone only minor differentiation.
Parental magmas may be primary or primitive. The only requirement is that they lead to magmas of other compositions. If a collection of melts with different compositions evolve from the same parent, they form a magma series. Although the melts have different compositions, they will share some chemical characteristics, especially trace element compositions and isotopic ratios. A challenge for petrologists is to study the compositions of an inferred magma series to learn the composition and source of the original parent.
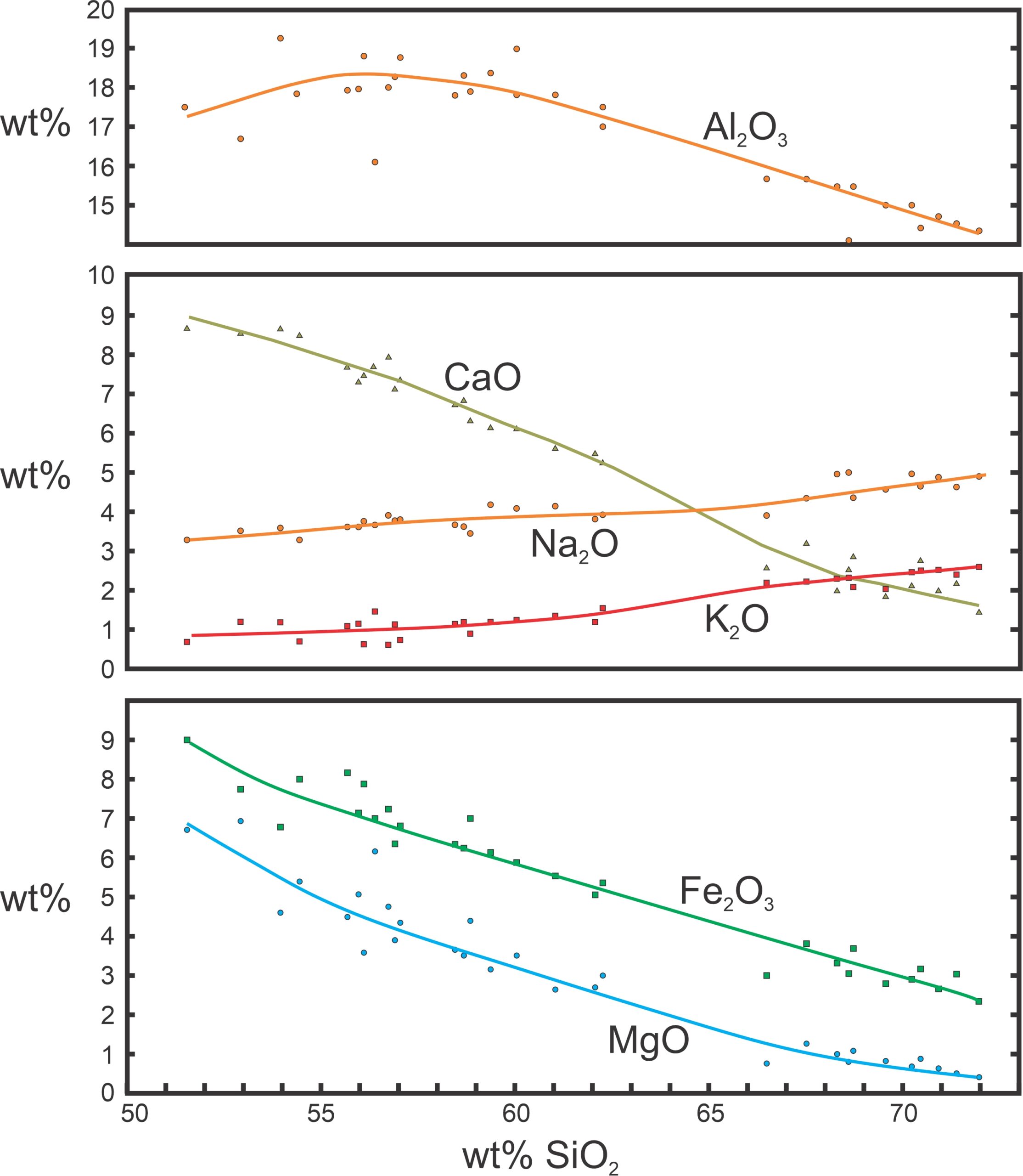
Typically, petrologists begin their quest by obtaining analyses of the rocks and plotting the results on different kinds of composition diagrams. For example, Harker diagrams, first used in 1902, have SiO2 content as the horizontal axis and other oxides plotted vertically (Figure 3.40). SiO2 is chosen as the abscissa because it generally shows the most variation of all oxides, and because it relates closely with magma temperature and the amount of fractional crystallization.
When looking at Harker diagrams, the principles are that (1) if derived from a common parent, rock compositions should trend smoothly across a diagram; and (2) the most mafic composition is closest to the parent magma composition. So, if a Harker diagram reveals smooth trends, it is possible that all the magmas derived from the same parent and that the low SiO2 end of the graphs are closest to the magma’s parent composition. Harker diagrams are only one kind of composition diagram; many others with different oxides on the axes are commonly used.
Figure 3.40 shows a well-studied Harker diagram for volcanic rocks from near Crater Lake, Oregon, based on the data of Howell Williams (1942). Each point represents a different volcanic rock from the same region; the horizontal axis shows the SiO2 content of the rock and the vertical axis the amount of other oxides present. The solid lines show the smoothed trends. The smooth trends are evidence that the different rocks may have derived from the same original parental magma. The Crater Lake magmas range from basalt (on the left side of the diagram) to rhyolite (on the right side). Based on the trends shown, Williams concluded that the magmas all came from a common parent magma and that they evolved by fractional crystallization. The basalt composition is closest to that parent.
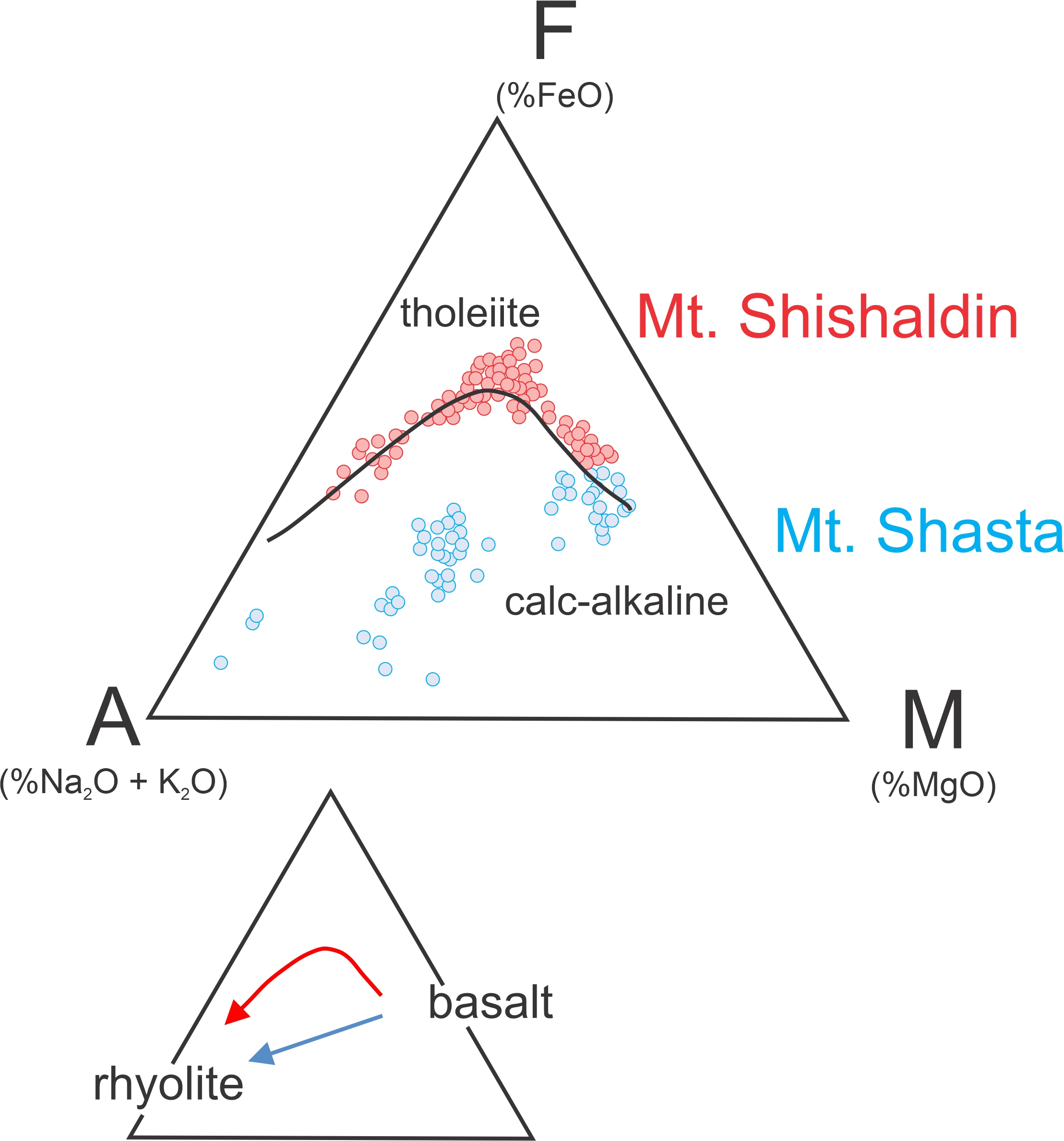
A second commonly used way to look at magma composition is to plot compositions on an AFM diagram (Figure 3.41). AFM diagrams ignore SiO2 and instead look at alkali (Na2O + K2O), iron as FeO (assuming it is not Fe2O3), and MgO content. The triangle corners are: A = alkali oxide weight %, F = FeO weight %, and M = MgO weight %. Many studies have found that magma series follow one of two trends, the tholeiite trend or the calc-alkaline trend, and we easily see these on an AFM diagram. Figure 3.41 is an AFM diagram comparing rocks from Shishaldin Volcano (Aleutian Islands) and Shasta Volcano (California). Each point represents an analysis of an individual rock. Shishaldin is an island arc volcano associated with an oceanic plate subducting under another oceanic plate. Shasta is a continental margin volcano where an oceanic plate is subducting under a continental plate. The Shishaldin data follow a tholeiite trend, depicted by the solid line and red arrow that initially moves toward the F-corner before curving downward toward the A-corner. The Shasta data follow a calc-alkaline trend (depicted by the blue line that heads directly toward the A-corner).
Whether tholeiitic or calc-alkaline, originally mafic magmas can produce rocks ranging from basalt to rhyolite, as the bottom triangle in Figure 3.41 shows. At both Shishaldin and Shasta Volcanos, more primitive parental magmas were mafic and the later evolved magmas were silicic. They differ, however, because tholeiitic magmas become iron-rich as they evolve, moving initially toward the F apex of the triangle. Calc-alkaline trends go directly from basalt to rhyolite.
The trends on an AFM diagram reveal clues about the environments in which the magmas differentiated. The difference between calc-alkaline and tholeiite trends is due to the oxidation state of iron. If iron is mostly oxidized, magnetite (Fe3O4), a mineral that contains oxidized iron (Fe3+), crystallizes early from a melt. If the iron is mostly reduced (existing as Fe2+), magnetite does not crystallize. In calc-alkaline magmas, the iron is oxidized, leading to crystallization of magnetite. Consequently, when mafic minerals crystallize, iron is removed from the magma as fast as magnesium and the melt’s Fe:Mg ratio remains about constant during differentiation. In tholeiitic magmas, olivine and pyroxene crystallize first and magnetite may not crystallize at all. Olivine and pyroxene have high Mg/Fe ratios compared with melt, and the magma becomes enriched in iron during the initial stages of crystallization. Calc-alkaline magmas are dominant in andesitic-type subduction zones, such as California’s Cascade Mountains. Mt. Shasta is an example. Tholeiitic trends occur mostly in island arcs, such as the Aleutian Islands, and Shishaldin Volcano is an example.
3.8 A Closer Look at Magma Chemistry
3.8.1 Major and Minor Elements
Major elements, typically present at levels exceeding 1 weight %, determine most magma properties and eventually the minerals that may form. Typical major elements in igneous rocks include O, Si, Al, Fe, Ca, Na, K, and Mg. In some rocks, Ti, Mn, P, and perhaps others may be considered major elements. Rocks also contain minor elements. Minor elements, typically comprising 0.1 to 1 weight % of a magma or rock, substitute for major elements in minerals but are selective about which minerals they enter. For the most part, they do not affect magma properties.
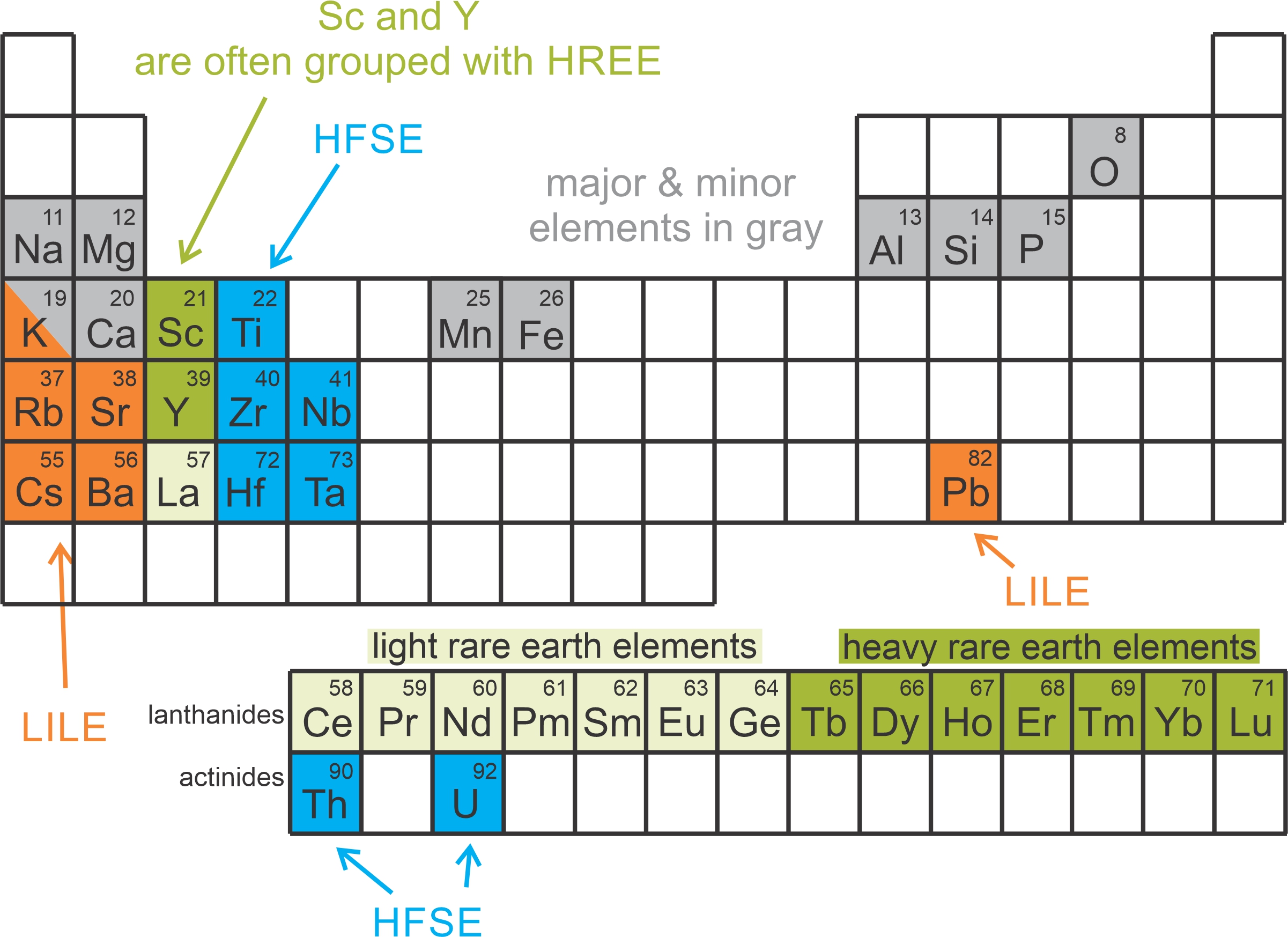
Figure 3.42 shows some key elements in igneous rocks. Major and minor elements are shaded gray. The other shaded elements are incompatible elements in mafic minerals (discussed in detail below). The incompatible elements include large ion lithophile elements (LILE) shaded orange, heavy rare earth and related elements shaded dark green, light rare earth elements shaded light green, and high field strength elements (HFSE) shaded blue. Potassium (K) is considered both a major element and an LILE element.
Trace elements are elements present in very small amounts, amounts even smaller than amounts of minor elements. The amount of any trace element that can enter a growing crystal depends mostly on ionic charge and radius. Some trace elements enter growing crystals in the early stages of crystallization, but others may remain in a magma until the latest stages of crystallization. Trace elements are even more selective than minor elements about the minerals they enter and generally have insignificant effects on rock and mineral properties. Because trace elements are present in very small amounts, petrologists commonly report them in parts per million (ppm) or parts per billion (ppb) instead of weight % (wt %). 1 ppm = 0.0001 wt %. Furthermore, when plotting trace element analyses, petrologists normalize the raw data by dividing by the composition of some reference standard. Normalization means that the range of values becomes small enough so that we may plot all trace elements on a single graph.
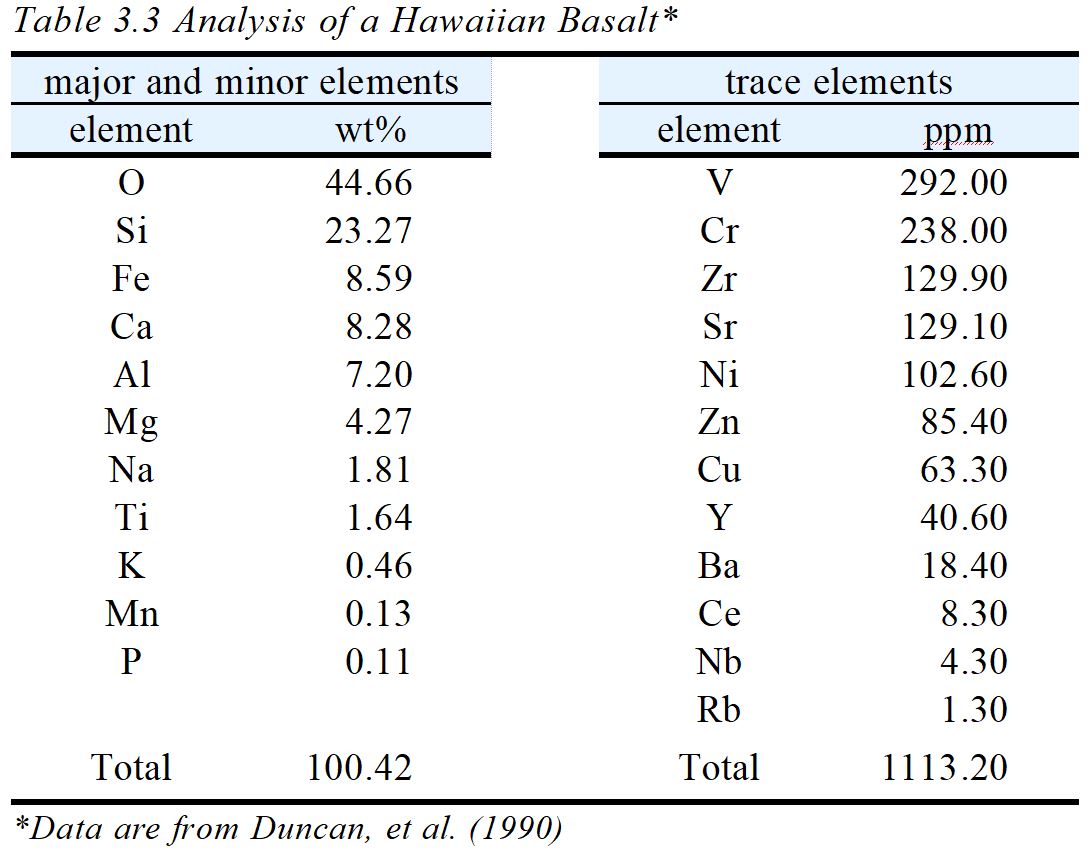 Table 3.3 contains an analysis for a Hawaiian basalt. The analysis does not distinguish between major and minor elements (because the distinction between the two is a fuzzy one), but Mn and P together comprise only about 0.2 weight % of the rock and would be considered minor elements by most petrologists. K, too, might be considered a minor element in this rock. The analysts listed trace elements separately, reporting them in ppm instead of weight %, and the total of the trace elements is about 1,113 ppm, which is equivalent to 0.1113 weight %. Many other trace elements are undoubtedly present in the Hawaiian basalt, but were not analyzed because they were not important to the study being conducted. Although Mn and P are minor elements in the Hawaiian basalt (and most other basalts), they may be concentrated in other kinds of rocks. The alkalis, major elements in silicic rocks, may be nearly absent in mafic rocks, although in the Hawaiian basalt they add to about 2.25 weight %. So, minor elements in some types of rocks can be major elements in others and vice versa.
Table 3.3 contains an analysis for a Hawaiian basalt. The analysis does not distinguish between major and minor elements (because the distinction between the two is a fuzzy one), but Mn and P together comprise only about 0.2 weight % of the rock and would be considered minor elements by most petrologists. K, too, might be considered a minor element in this rock. The analysts listed trace elements separately, reporting them in ppm instead of weight %, and the total of the trace elements is about 1,113 ppm, which is equivalent to 0.1113 weight %. Many other trace elements are undoubtedly present in the Hawaiian basalt, but were not analyzed because they were not important to the study being conducted. Although Mn and P are minor elements in the Hawaiian basalt (and most other basalts), they may be concentrated in other kinds of rocks. The alkalis, major elements in silicic rocks, may be nearly absent in mafic rocks, although in the Hawaiian basalt they add to about 2.25 weight %. So, minor elements in some types of rocks can be major elements in others and vice versa.
We can classify and name igneous rocks based on the minerals they contain, but magmas, because they contain no minerals, must be classified in another way. Additionally, many volcanic rocks may contain glass instead of minerals or may be too fine grained for mineral identification. Thus we often classify magmas and many igneous rocks based on their chemical composition instead of their mineralogy.
Although igneous rock chemistry varies in many ways, the silica content and alkali content of volcanic rocks form the basis for one of the most commonly used classification schemes. It is not that other compositional variations are unimportant, but that many other possible variations correlate with alkali and silica content and so the classification system captures well the variation in rock compositions.
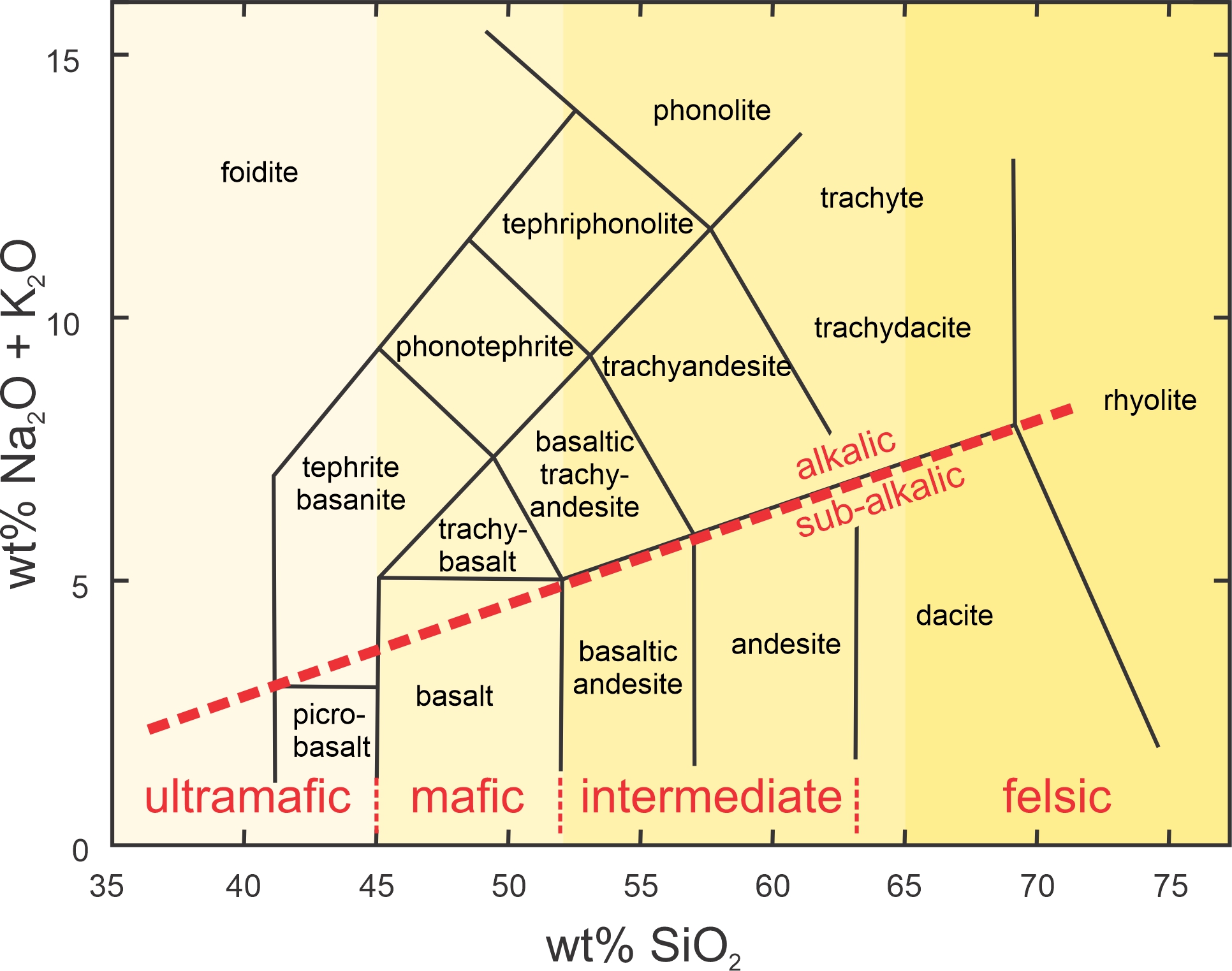
Using the total alkalis versus silica (TAS) system (Figure 3.43) is straightforward, and the weight percentages of silica (SiO2) and alkali oxides (Na2O + K2O) in a rock are used to obtain a rock name. The vertical axis is the total alkali oxide content, and the horizontal axis is the silica content. In the TAS diagram, ultramafic compositions (low silica content) plot on the left and silicic compositions (high silica content) on the right, with mafic and intermediate compositions between. The diagonal red line divides the diagram into two parts. Compositions that plot in the upper part of the diagram are alkalic; they are relatively rare in nature. Those plotting in the lower part of the diagram are termed sub-alkalic and are much more common. By far, the most common volcanic rocks are sub-alkalic: basalt, andesite, dacite, and rhyolite. Although the names in Figure 3.43 are names of volcanic rocks, they are often used to describe magma types. A dacite magma, for example, is one that could erupt to form a dacitic rock.
Although both silica and alkali content are keys when classifying magmas or volcanic rocks, silica content alone explains many variations in magma properties (Table 3.4). It is also the basis for a rock-naming scheme that is simpler than the TAS system is: simply calling rocks ultramafic, mafic, intermediate, or silicic. Variations in magma properties with silica content are profound. The more silicic a magma, the lower its eruption temperature but the greater the possibility for explosive eruptions due to high viscosity and gas content. Note that in this simpler classification scheme, alkali content correlates with silica content. This correlation is not always the case, as shown in the TAS diagram, but is the case for sub-alkalic rocks. Similar to the names in the TAS system, the rock names in Table 3.4 are often used to describe magma composition. For example, we might describe a magma as granitic if it could crystallize to form a granite.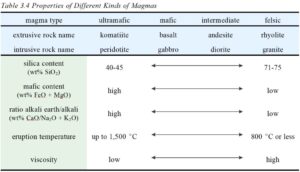
3.8.2 Incompatible and Compatible Elements
We can divide elements in igneous rocks and magmas into two groups: those that tend to remain in a magma until the later stages of crystallization (and consequently become enriched in the magma as crystallization takes place), and those that are easily incorporated into early growing crystals (and consequently become depleted in a magma quickly). Elements that tend to remain in the magma are said to be incompatible, and those that enter crystals quickly are compatible. Petrologists use these terms, incompatible and compatible, most commonly to describe trace elements, but they apply equally well to major and minor elements. Some elements behave as compatible elements in some magma types, but incompatible in others, because the specific minerals that crystallize vary with magma composition. Trace elements, however, both compatible and incompatible, are especially useful as trackers of magma evolution. Incompatible elements include the rare earth elements, elements 57 through 71 (La – Lu), but their degree of incompatibility varies with atomic number. The rare earths, and other incompatible elements are highlighted in the periodic chart of Figure 3.42.

Incompatible elements fall into two main groups, a group that has large ionic radius, and a group that has large ionic charge (Figure 3.44). The first group includes alkali and alkali earth elements, notably K, Rb, Cs, Sr, Ba, and several other elements. Elements that tend to concentrate in Earth’s crust and mantle are called lithophiles. So, the alkalis and alkali earths, and elements with similar properties, are collectively termed large ion lithophiles, or LILE for short (Figure 3.44). LILEs do not fit into crystallographic sites in most minerals, and the charge attracting them to growing crystals is small, so they tend to remain in a melt.
Elements that form ions with small radii and high charge are called high field strength elements (HFSE) (Figure 3.44). This group of incompatible elements includes Zr, Nb, Hf, Th, U, and Ta. HFSEs have high ionic charge (+4 or greater), which means that, if they enter crystals charge balance is hard to obtain. Consequently, HFSEs tend to remain in a melt. For some purposes, HFSE are defined as those whose ions have a charge to radius ratio greater than 2 (to the right of the dashed line in Figure 3.44).
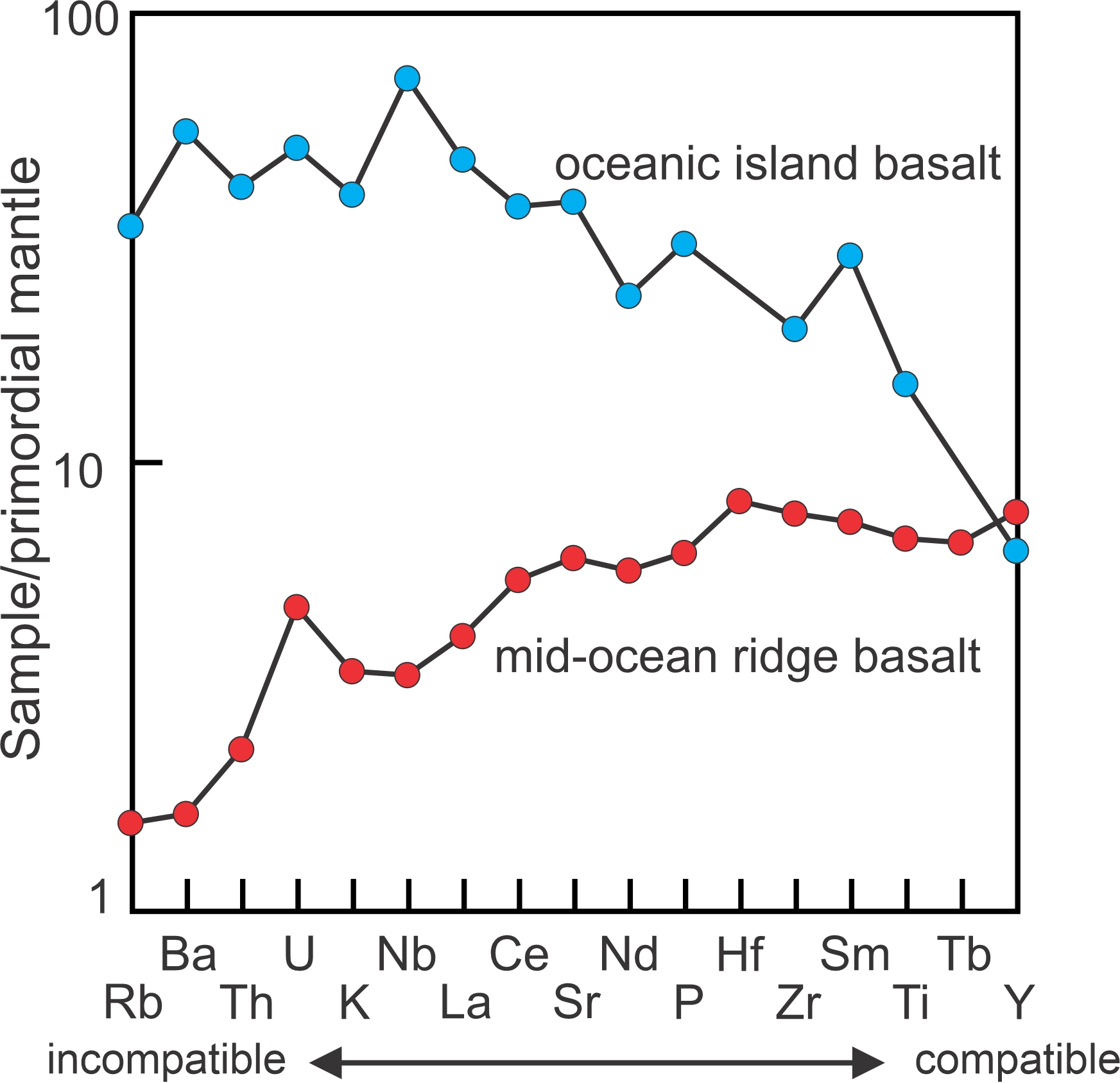
Figure 3.45 compares the trace element content of an oceanic island basalt with that of a mid-ocean ridge basalt. In diagrams of this sort – sometimes called spider diagrams – the most incompatible elements are on the left, and elements are increasingly less incompatible moving to the right. The mid-ocean ridge basalt is depleted in incompatible elements compared with the oceanic island basalt. However, both have about the same concentrations of the compatible elements. This diagram, therefore, suggests that mid-ocean ridge magmas, associated with active plate spreading centers, derive from regions that have undergone significant amounts of partial melting. In contrast, the oceanic island magmas come from regions that are more primitive.
Evidence of this sort allows petrologists to conclude that the upper oceanic mantle – the source region for mid-ocean ridge basalts – is an area of active melting and recycling of material. The source of oceanic island basalts, such as those that reach the surface in Hawaii, is deeper in the mantle where melting has not removed incompatible elements. Note that the data for both basalts were normalized by dividing the analyses by an estimated composition for the primordial mantle (to keep numbers on scale). Additionally, the vertical scale is a log scale; if it were not, the trends would not be as easily seen.
Some transition elements – including nickel, cobalt, chromium, and scandium – are also important trace elements. They have small radii, and (usually) +2 or +3 ionic charge, and are incorporated into mafic minerals during the earliest stages of crystallization and tend to remain there. So, they are compatible when melting occurs in the mantle and, consequently, melts from the mantle contain them in very low amounts. They are often used as markers that determine where in Earth a magma originated.
The concept of compatible versus incompatible elements depends on rock type, because different rocks contain different minerals that incorporate elements in different ways. Scandium may enter pyroxenes, but not enter olivine. Zirconium is easily accommodated in zircon. Phosphorus concentrates in apatite, but neither zirconium nor phosphorous go into olivine. Earth’s mantle is primarily composed of olivine and pyroxene, and these minerals become enriched in scandium, nickel, titanium, chromium, and cobalt as fractional melting occurs. So, melts derived from the upper mantle are enriched in these elements, and the amount of melting that has occurred can be estimated based on trace element abundance.
3.8.3 Rare Earth Elements
The lanthanide elements, also called the rare earth elements (REE), having atomic numbers 57 (La) to 72 (Lu), are very important trace elements with high field strength (Figure 3.44). Elements 57 through 64, La through Gd, are considered light REEs; 65-71, Tb through Lu, are heavy REEs. Y and Sc are sometimes grouped with the heavy REEs due to similar properties. The REEs all have a large ionic radius and, except Eu, they are trivalent (+3). Ionic radius decreases with increasing atomic number, so La and other light REEs (largest) are more incompatible than Lu and other heavy REEs (smallest) are. If garnet crystallizes, it incorporates heavy REEs (Ho-Lu) easily, making them especially compatible.
During fractional crystallization of a magma in the mantle or the crust, incompatible elements stay preferentially in melts and, so, tend to move up and concentrate in Earth’s outer layers. The original source region is fertile if there has been little removal of incompatible elements, but fertile rocks become depleted rocks when melting occurs. Rocks or magmas that are rich, or only slightly depleted, in light rare earth elements are fertile, and those with strong depletions in LREE are depleted, because their chemistry indicates the degree to which they have melted. This is why trace elements are powerful indicators of magma origin. Magmas that originate in upper levels of Earth are richer in incompatible elements than those that come from pristine mantle sources. Additionally, petrologists distinguish both fertile and depleted magma source regions within the mantle.
When a rock melts, the incompatible elements enter the melt quickly. Consequently, the concentration of incompatible elements will be very high after just a little bit of melting. With more melting, the concentrations decrease because most incompatible elements are already in the melt and get diluted with further melting. This effect is especially true for elements present in rocks and magma in very small amounts, which explains why trace elements are such powerful indicators of magma origin. So, trace elements tell us how much melting has occurred to produce a magma and, although a bit more complicated, they also monitor how much crystal fraction occurs when a magma cools.
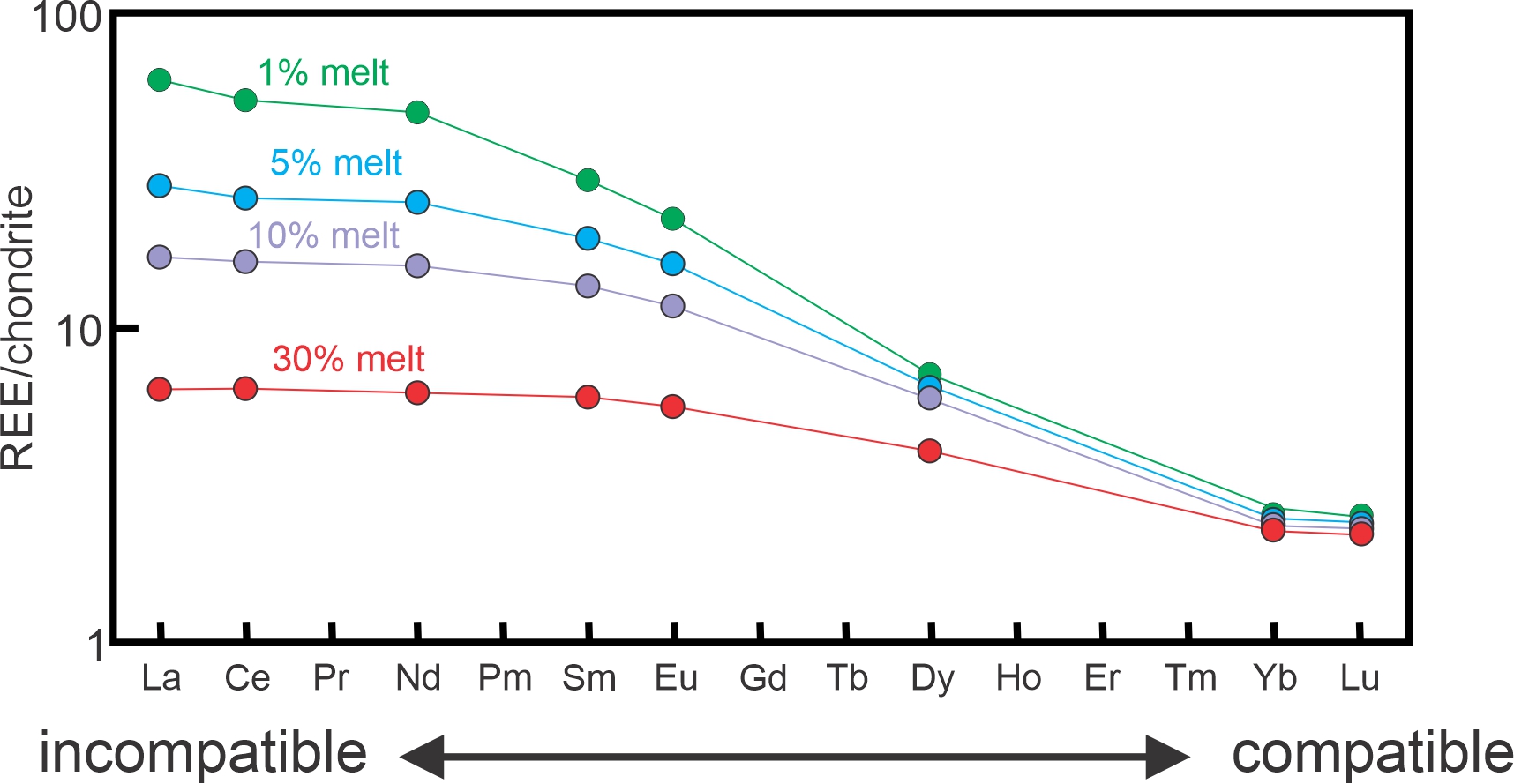
Figure 3.46 shows rare earth patterns for melts derived from a garnet peridotite. If only a small amount of melting occurs, concentrations of incompatible elements are high because they enter the melt first. With increased melting, other elements enter the melt and the concentrations of incompatible elements goes down. In this diagram, the compositions were normalized by dividing by the standard composition of a chondritic meteorite, and the scale is a log scale.
● Figure CreditsUncredited graphics/photos came from the authors and other primary contributors to this book. 3.0 (opening) Skylight, Kilauea Volcano, United States Geological Survey (USGS) |
Contents
- 3.1 Volcanism in Yellowstone National Park
- ● Box 3.1 Geothermal Features in Yellowstone National Park
- 3.2 Magma Compositions
- 3.3 Magma Sources
- 3.4 Magma Movement
- 3.5 Melting of Minerals and Rocks
- 3.6 The Importance of Partial Melting and Fractional Crystallization
- 3.8 A Closer Look at Magma Chemistry
- ● Figure Credits