9 Ore Deposits and Economic Minerals
KEY CONCEPTS
- We mine many minerals from Earth.
- Some ores are valued for their mineral properties, some for the elements they contain, and others because they contain valuable gems.
- The best ore deposits are those containing large amounts of ore minerals.
- The best metal ore minerals are those that contain large amounts of metals of value.
- Most metallic ore minerals are native elements, sulfides, sulfosalts, oxides, or hydroxides.
- Ore deposits are highly variable in nature and origin.
- The most important kinds of ore deposits are magmatic, hydrothermal, and sedimentary.
9.1 Mineral Commodities
9.1.1 Mineral Resources
Earth gives us many mineralogical resources, also called mineral commodities. Fewer than a dozen minerals and eight or nine elements dominate the crust – we use most of them in our daily lives. Other elements and minerals that exist only in small amounts and have uneven distributions, are equally vital. We mine some ores because they contain elements that have the metallic properties of conductivity, strength, or shiny appearance. We mine industrial minerals such as halite, gypsum, clays, calcite, asbestos, micas, and zeolites to make salt, plaster, ceramics, construction materials, electronic components, chemical filters, and many other things. We also quarry large quantities of limestone (to make cement) and building stone, and energy companies produce large amounts of coal, oil, gas, uranium and other energy resources.

We mine diamond and other potential gem minerals for jewelry and also for use in industry. The photo in Figure 9.2 shows the Diavik Diamond Mine in remote Canada. This mine has produced about 10 million carats of rough diamond since it began operations in 2003.
When we think of diamonds, we generally think of gems, but gem diamonds are rare. Most natural diamonds, called industrial diamonds, have little gem quality. Because of diamond’s great hardness, industrial diamonds have many important uses. Most commonly they are used as an abrasive or polishing agent. But they are also incorporated into grinding wheels, saw blades, and drill bits used to manufacture products from very hard materials. Thus, diamonds and many other mineral commodities are used in many different ways.
Ore deposits and ore minerals fall into several main commodity groups: metallic and semimetallic elements, nonmetallic elements, gems, construction and manufacturing materials, fertilizer and chemical minerals, and energy resources (table below). We take energy resources and construction materials from Earth in the greatest quantities. We also mine large amounts of salt and fertilizer components. Of the metals, only iron is removed from Earth at rates comparable to these components.
| Groups of Mineral Commodities | |
| group | examples |
| metallic and semimetallic elements | gold, silver, copper, iron, manganese, aluminum |
| nonmetallic elements | potassium, sodium, phosphorous, sulfur |
| gems | diamond, sapphire, agate |
| industrial materials: construction and manufacturing | sand, clay, building stone, diatomite, talc, mica, zeolites |
| industrial materials: fertilizer and chemicals | limestone, phosphate, potash, salt, nitrate, fluorite |
| energy resources | coal, oil, gas, uranium |
Many different mineral commodities are important to modern society. However, when mineralogists think about mining, they are generally thinking of ore minerals that are the sources of important metals, or of minerals that have specific, highly valued properties (e.g., asbestos, micas, potash, and gems of all sorts). That is what we will focus on in most of the rest of this chapter.
9.1.2 The History of Mining
Mining is nothing new. People have practiced mining and quarrying since ancient times. The first mineral known to be mined was flint, a fine-grained variety of quartz used to make weapons. Early peoples mined other things, such as ochre, for use as pigment in art and religious ceremonies.

Archaeologists and anthropologists define major periods of human civilization based on resources used. There is a great deal of overlap and different regions moved from one period to another at different times. The late Stone Age, also called the Neolithic Age, was followed by the Chalcolithic Age from 4,500 to 3,500 BCE (Before Common Era). During the Chalcolithic Age, humans began using copper (the name chalcolithic is derived from the Greek word khalkos for copper) for both decorative and utilitarian purposes. One of the largest collections of chalcolithic artifacts was discovered in a cave in Israel’s Judean Desert in 1961 (Figure 9.3). Because copper could be found as malleable pure copper nuggets, people could shape it with available stone tools – a property that no other common minerals possessed. A rise in consumption of copper coincided with the development of a socioeconomic hierarchy, and the wealthy citizens possessed more copper than the proletariat.

Figure 9.4 shows artifacts from the Bronze Age, the age that followed the Chalcolithic Age and lasted from about 4,200 to 1,000 BCE. Use of bronze first developed in the Mesopotamian civilization of Sumeria and became common in other places later. This was a period characterized by a rapid rise of resource consumption and increasing diversification of products made by metalworking. Perhaps the most significant advancement in metal use was the discovery of how to make bronze, an alloy created by melting and combining the metals copper and tin. Although tin melts at a relatively low temperature (232 °C), copper melts at 1,983 °C, a temperature too great to be easily achieved at the time. However, clever metal workers discovered that a mix of one part tin and three parts copper melts at 1,675 °C, which was low enough to make bronze manufacturing possible in many places.
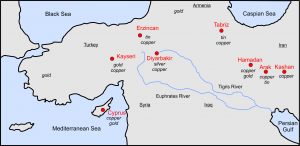

The map in Figure 9.5 shows the location of Bronze Age mines that provided metals used throughout much of the Middle East. Most of the copper came from the Troodos Mountains of Cyprus, where copper could be found in loose sediments at Earth’s surface. Even today, a great deal of copper mining takes place in Cyprus, although most operations have moved underground. Figure 9.6 shows the Skiriotissa Mine, which was the site of Bronze Age mining and is an active mine today. Although some copper and other metals came from mines on Cyprus and on the Asian mainland, tin deposits were generally small or hard to produce. The scarcity of tin in the Middle East and other areas of the Mediterranean region, meant that tin ores came from as far away as the British Isles, which the Greeks named the Cassiterides, that translates to Tin Islands.
A key property that allowed humans to work with bronze is that, after pouring the molten bronze into stone molds and allowing the liquid to cool to a solid, the copper-tin alloy could be formed and shaped using hammers at room temperature, a process called cold working. And, because bronze is much stronger than copper, people could make many improved products, including knives, shields and swords, and tools that led to more productive agriculture.

The Iron Age followed the Bronze Age beginning around 1500 BCE, when the Hittite society of ancient Anatolia (modern day Turkey) discovered how to process iron. Their technological breakthrough was to add a small amount of charcoal (carbon) to rocks that contained iron. Pure iron melts at 1,538 °C, but adding carbon results in a carbon-iron mixture that melts at 1,170 °C. The Hittites also figured out that iron-carbon alloys could not be cold-worked like bronze but had to be hammered and shaped while hot. Thus, they invented the art of modern blacksmithing. The iron and alloys produced, once cooled, were much stronger and harder than bronze was. After the time of the Hittites, it took another 500 to 1,000 years for the iron age to reach central and northern Europe (Figure 9.7).
The source of iron used by the Hittites was metallic meteorites. Meteorites also contained a small amount of nickel that improved metal properties. Because iron-rich meteorites were not in abundance, the Hittites carefully guarded their invention of iron metal working for several centuries. During those centuries, the Hittites exercised military superiority over much of the Middle East and Egypt, where the weaker bronze was used in battle. However, by 1200 BCE, iron metal working technology had spread across the Middle East, North Africa, Europe, and to Asia; people discovered new sources of iron; and the Hittite empire disappeared.

Egyptians mined native metals, including gold, silver, and copper, from stream beds as early as 3700 to 3000 BCE. Around 2600 BCE they began to quarry stone to build the Great Pyramids. By the Middle Ages, mining was common in Europe. Georgius Agricola (Figure 9.8), a German physician, wrote the first widely read book about mining, De re metallica, published in 1556. Agricola’s work is said by some to represent the beginning of the science of mineralogy.
Mineral resources literally put places on the map of the ancient world. If a region contained abundant amounts of copper, silver, tin, or gold, and later iron, it soon became populated and prosperous. Civilizations established trade routes and developed commercial systems, shipping commodities over increasingly longer distances. If resource supplies became depleted in one location, people sought new sources. Thus, exploration was needed to sustain production and consumption of valuable resources. These same dynamics operate today: when new mineral deposits are discovered, new communities and industries may appear. When old deposits become depleted, communities and industries wane. And, always, mining companies are exploring to find new sources of economically viable minerals.
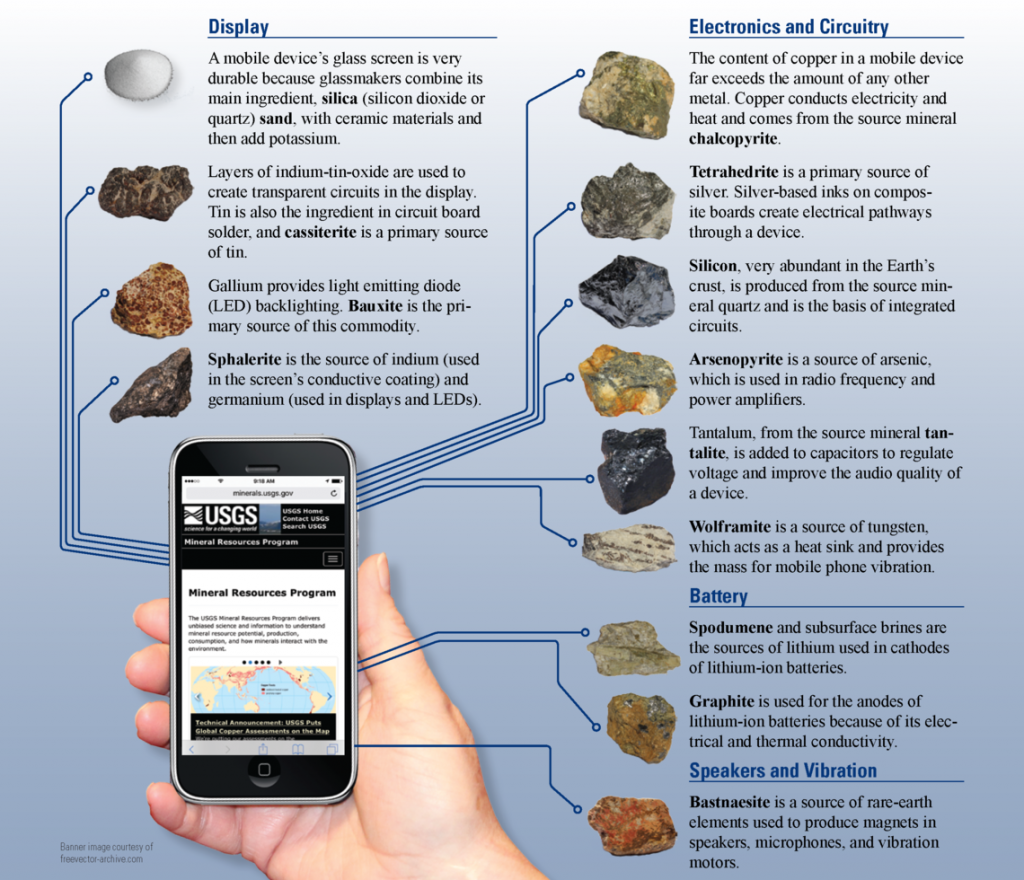
Copper, tin, iron, and nickel were all important during the early ages of humans, and they are equally important today. Those same metals – and many others – are key parts of a seemingly infinite number of products. For example, Figure 9.9 (below) shows the many minerals that provide elements that are in a smart phone. Copper makes up about 10% of the weight of a smartphone, and that copper is the key to moving around the electricity that powers the phone. Tin is used to make the liquid crystal display (LCD) screen and to solder electrical connections that transmit digital information. Iron is combined with the metals neodymium and boron to make magnets that are part of the microphone and speaker. And those are not the only elements in a smartphone; there are about 75 elements in all. Without any one of these elements, smartphones would not exist as they do. Nearly everything that we manufacture contains mineral resources, and the sources for these resources are mineral deposits.
9.1.3 Mineral Deposits, Ore Deposits, and Mining
A mineral deposit is a place in Earth’s crust where geologic processes have concentrated one or more minerals at greater abundance than in the average crust. An ore deposit is a mineral deposit that can be produced to make a profit. Thus, all ore deposits are mineral deposits, but the reverse is not true.
Many factors control the profitability of an ore deposit. We call the amount of known ore in a deposit the reserves. The concentration of a commodity in the ore (the “richness” of the ore) determines the ore grade. When calculating the profitability, amount of reserves and ore grade are the most significant geological factors, although economic factors such as extraction costs, processing costs, and market price are often more decisive. A high-grade ore deposit may be uneconomical to mine if the reserves are low, because start-up costs could consume all profits. A large high-grade deposit may be uneconomical to mine if it is in a remote area. Even large, developed deposits can become uneconomical if the market price falls, perhaps due to the discovery of a better deposit somewhere else.

Mining operations have many guises. Surface mining involves uncovering resources by removing overburden. This is done in several ways but, for metal deposits, open pit mining is most common. The Bingham Canyon Mine (Figure 9.10, above), near Salt Lake City, is the world’s deepest open pit. It is 4.5 km by 1.3 km in area and is 1.2 km deep. Since mining began just over 100 years ago, the Bingham Canyon open-pit mine has produced 24 million tons of copper, 790 tons of gold, 6,600 tons of silver, and 425,000 tons of molybdenum. To put these numbers in perspective, consider that, in total, the people of the world consume about 30 million tons of newly mined copper, 2,750 tons of newly mined gold, 30,000 tons of newly mined silver, and 250,000 tons newly mined molybdenum every year. So, the cumulative amounts of copper, gold, silver and molybdenum mined from Bingham Canyon over an entire century are insufficient to satisfy peoples’ needs for even one year. Thus, to maintain our societies and lifestyles, without recycling metals, the world’s people need many mines like the one at Bingham Canyon.

When open pit mining cannot get to valuable resources, mining may move underground. Underground mining involves digging tunnels and shafts to reach buried ore bodies. This takes on many forms depending on the nature of an ore deposit. The Mponeng Gold Mine (Figure 9.11), southwest of Johannesburg, South Africa, is the deepest mine in the world. Its deepest workings are more than 3.5 km below Earth’s surface. Figure 9.11 shows the mine buildings on the surface; the tall towers are head frames where lifts descend to depth where the actual mining takes place.

After mining, processing separates and concentrates valuable minerals from the ore. This involves crushing the ore rock, followed by gravity and chemical separation. Any unwanted rock and minerals, called waste rock and gangue, respectively, are usually discarded in tailings piles. This photo (Figure 9.12) shows tailings at a mine just south of Quebec City, Quebec. Besides piling discarded material on the surface, sometimes miners return wastes to abandoned portions of a mine to fill voids left by ore removal.

Mining commonly comes with some significant environmental costs because disposal of mine waste can lead to significant problems. And, abandoned mines also pose environmental problems. Runoff from waste piles or mine sites may create soil, groundwater, or surface water contamination. Many ore bodies, for example, are rich in sulfide minerals. After mining ceases, sulfur from the minerals can react with water and air to create acid mine drainage (Figure 9.13) or acid runoff from tailings piles. The resulting sulfuric acid may kill vegetation and fish in nearby lakes and streams. Contamination may also occur because of non-natural chemicals used during mineral production. And, there may be terrain costs. After mining ceases it is often impossible to restore the mined land to anything like its pre-mining condition. Open pits remain forever and so do tailings piles. Mining sometimes also leads to increased erosion or the formation of sinkholes.

Most mining companies are responsible and do their best to reduce impacts while mining takes place, but eliminating them all is impossible. And, many mining companies do a good job of cleaning up after they are done. The photo seen in Figure 9.14 shows a site near Cannock Chase, England, that was once a coal mine. The area was restored and is now used for picnicking, walking and cycling. The United States, like many other countries, has enacted laws to limit the damage caused by mining. These laws, however, are often controversial because they may drive up the price of mined commodities. Communities that rely on mining for employment, in particular, worry that regulations may lead eventually to loss of jobs.
9.1.4 Natural Abundances of Elements
Oxygen, silicon, aluminum, iron, calcium, sodium, potassium, magnesium, and titanium make up 99% of Earth’s crust. We saw a histogram of this distribution in Figure 2.2 (Chapter 2). It is no wonder, then, that humans have developed ways to use these elements in industry, agriculture, and manufacturing. Less abundant elements have also become important to modern society. These include metals, radioactive elements such as uranium or thorium, and fertilizer components including, most importantly, nitrogen and phosphorous. As shown in the table below, some important elements make up very small percentages of Earth’s crust; nevertheless, natural processes concentrate them in particular minerals and in particular places.
| Natural Abundance, Economical Ore Grade, and Concentration Factors for Some Metals | |||
| ore resource | metal concentration in average crustal rock | minimum ore grade for profitable extraction | economical concentration factor |
| aluminum | 8.2 wt% | 30 wt% | 4 |
| iron | 5.6 wt% | 20 wt% | 4 |
| sodium | 2.4 wt% | 40 wt% | 17 |
| manganese | 0.09 wt% | 35 wt% | 370 |
| chromium | 0.01 wt% | 30 wt% | 2,940 |
| nickel | 0.008 wt% | 0.5 wt% | 60 |
| zinc | 0.007 wt% | 4.0 wt% | 570 |
| lead | 0.001 wt% | 4.0 wt% | 2,900 |
| copper | 0.006 wt% | 0.5 wt% | 80 |
| tin | 0.0002 wt% | 0.5 wt% | 2,500 |
The economical concentration factor listed in the table above is the ratio of typical minimum economical ore concentration to average crustal concentration. For example, the average crustal abundance of chromium is about 0.01 wt %. Chromium ore can sometimes be profitable if it contains 30 wt % chromium. The necessary concentration factor is therefore nearly 3,000 – chromium must be concentrated at least 3,000 times to create profitable ore. The table compares economical concentration factors for a dozen different metals. They are ordered from those most abundant (top) to those that are rare (bottom). Concentration factors range from 4 for aluminum and iron, to nearly 3,000 for tin, chromium and lead.
Elements that occur in high abundance do not need a high concentration factor to make mining economical. In contrast, less common chromium, lead, tin, and zinc require great concentrations to be profitably mined (see the table above). We mine relatively common elements, such as iron and aluminum, in many places worldwide; we mine rarer elements, including tin, chromium, or lead, in far fewer places.

Although the table does not include prices, there is a correlation between the economical ore grades and the price of a given resource. Gold, for example, is much more expensive than the metals listed, although the demand for gold is less than for the others. This price difference exists because the natural processes that concentrate most commonly used metals are much more common than the processes that concentrate gold, so there are fewer high-quality gold deposits than there are other kinds of deposits. Many gold mines can remain profitable even if the ore contains less than 0.1 ounces of gold in a ton of rock. Figure 9.16 shows an example of gold ore from Greenland. The gold flakes are small – the entire photo is less than 2 cm across.
The market for metals can be extremely volatile. Geopolitics, wars, economic sanctions, and other things may cause major market disruptions. But, trends in technology may, over the long run, be even more significant. For example, beginning about 5 years ago, many predicted that the demand for electric vehicles (EV) was going to skyrocket. A growing EV industry means that demand for lithium-ion batteries will increase. So, in 2016, the average market price for lithium began rising and doubled in two years.
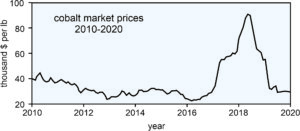
But, lithium-ion batteries also include other key metals besides lithium, for example cobalt. Between 2016 and 2018, cobalt prices increased from just over \$20,000 to \$95,000 per tonne (Figure 9.17). But, since spring of 2018, prices have collapsed as cobalt lost 75% of it value. Why did this happen? Several things are undoubtably important. Perhaps most significant is that the projected increase in EV sales and demand for lithium-ion batteries did not occur as rapidly as predicted. Additionally, when things looked good, the world’s major cobalt mines, most of which are in the Democratic Republic of the Congo, greatly increased production. At the same time, smaller independent operators started new mines.
So now we have a market surfeit of cobalt, and prices are about the lowest they have been in a decade. Still, market prognosticators say that with the inevitable increase in demand for EVs, and for rechargeable batteries in general, prices for cobalt, nickel and graphite, and other key components of lithium-iron batteries can be expected to increase soon.
9.1.5 Worldwide Distribution
Geological processes that concentrate minerals are not unusual. But, the processes that create economically productive ore deposits are rare. If they were not, market prices would fall, decreasing profits and putting some mines out of business. Thus, the largest and most easily produced mines control market prices. Old mines shut down and new mines open up when new discoveries are made. Today, however, new discoveries are generally smaller than in the past because the largest deposits, which are more easily found than smaller deposits, have already been developed.

Because the geology of Earth varies, the distribution of ore deposits around the globe is uneven, and the minerals industry flourishes in some places and not in others. Some regions of the world contain most of the supply of certain commodities; this can affect international politics. For example, the United States controls about half the world’s molybdenum. Figure 9.18 shows the Climax Mine in Colorado. This mine has historically been the largest producer of molybdenum in the world. Production started in 1915, but the mine temporarily shut down between 1995 and 2012 due to low molybdenum prices. Nonetheless, the US has sufficient supply of molybdenum. Unfortunately, many other important minerals are not produced in the United States. We call these minerals critical minerals, or strategic minerals (See Box 9-2). Additionally, we import many mineral commodities, that we might produce ourselves, because it would cost too much to mine them in our own country; tungsten is a good example.
Australia produces about a quarter of the world’s aluminum, and Zaire half the cobalt. China accounts for 95% of all rare earth production, although significant reserves are found in other countries. South Africa, a country rich in mineral commodities, controls 90% of the world’s platinum, half the world’s gold, and 75% of the chromium. Much of the world’s tin comes from Bolivia and Brazil.
South Africa is, arguably, the number one mining country in the world. Most South African ore deposits are associated with regions called Precambrian greenstone belts, ancient volcanic terranes. Similar greenstone belts in Canada account for many of North America’s metallic ore deposits. Figures 9.19, 9.20, and 9.21, above show rocks of greenstone belts in Africa, Canada, and Tanzania. Various other types of geological terranes are associated with ore deposits, too. Most economical metal and semimetal deposits are found near margins of continents, or the former margins of continents, where mountain building and igneous activity have occurred. Still, other types of deposits are found in continental interiors.
9.2 Ore Minerals
| Some Common Ore Minerals | ||
| metal | mineral | formula |
| Al | gibbsite boehmite diaspore |
Al(OH)3 AlO(OH) AlO(OH) |
| Fe | magnetite hematite goethite siderite pyrite |
Fe3O4 Fe2O3 FeO(OH) FeCO3 FeS2 |
| Cu | copper chalcopyrite bornite chalcocite covellite |
Cu CuFeS2 Cu5FeS4 Cu2S CuS |
| Ni | pentlandite garnierite |
(Ni,Fe)9S8 (Ni,Mg)3Si2O5(OH)4 |
| Zn | sphalerite wurtzite zincite franklinite |
ZnS ZnS ZnO ZnFe2O4 |
| Mn | hausmannite polianite pyrolusite cassiterite |
Mn3O4 MnO2 MnO2 SnO2 |
| Cr | chromite | FeCr2O4 |
| Pb | galena cerussite |
PbS PbCO3 |
| Ag | gold calaverite |
Au AuTe2 |
Ores and ore minerals vary greatly in quality. Ideal ores contain 100% ore minerals. Such ores do not exist, but some come close. Ideal ore minerals contain 100% of the commodity of interest. Native copper, for example, is an ideal copper ore mineral. Ideal ore minerals are, however, uncommon. The most commonly mined ores are not ideal. Instead they are rich in ore minerals that can be processed relatively inexpensively to isolate desired components.
The table seen here lists common ore minerals for various metals. The minerals include the native metals copper and gold, and many sulfides, oxides, and hydroxides. Minerals in these groups are generally good ore minerals because they contain relatively large amounts of the desired elements. Furthermore, processing and element extraction are usually straightforward and relatively inexpensive. That is why we mine Cu and Cu-Fe sulfides for their copper content and iron oxides for their iron content.
Silicate minerals, although common, are generally poor ore minerals and are not included in the table. For example, although aluminum is found in many common silicates, tight bonding makes producing metallic aluminum from silicates uneconomical. We obtain most aluminum from Al-hydroxides found in bauxite deposits.
We discussed igneous and sedimentary minerals in previous chapters. In the following section, we focus on economic minerals that belong to other groups.
9.2.1 Native Elements: Metals, Semimetals, and Nonmetals
| Native Metals | |
| gold | Au |
| silver | Ag |
| copper | Cu |
| platinum | Pt |
| Native Semimetals | |
| arsenic | As |
| antimony | Sb |
| bismuth | Bi |
| Native Nonmetals | |
| graphite | C |
| diamond | C |
| sulfur | S |
Native elements have high value because they may require no processing before being used in manufacturing, as currency, or for other purposes. The first metals ever used by humans were native minerals. Only later did humans develop refining techniques for the extraction of elements from more complex minerals. We conveniently divide native elements into metals, semimetals, and nonmetals based on their chemical and physical properties. The table to the right includes the most common minerals of each group.
Within the metal group, the principal native minerals are gold, silver, copper, and platinum. These four minerals all contain weak metallic bonds. Gold, silver, and copper have further commonality in their chemical properties because they are in the same column of the periodic table. Gold and silver form a complete solid solution; we call compositions containing both gold and silver electrum. But, because copper atoms are smaller than gold and silver atoms, solutions are limited between copper and the precious metals. Native gold, silver, and copper may contain small amounts of other elements. For example, native copper frequently contains arsenic, antimony, bismuth, iron, or mercury. Native platinum is much rarer than gold, silver, or copper. It typically contains small amounts of other elements, especially palladium. The native semimetals arsenic, antimony, and bismuth are also rare.
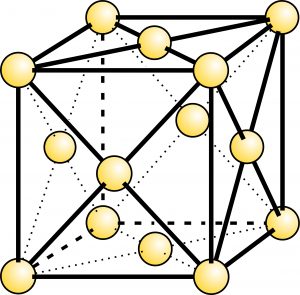
Native copper, gold, silver, and platinum have atomic structures with atoms arranged in a cubic pattern (Figure 9.23). Iron does, too, although native iron is rare, except in meteorites, and the atomic arrangement in native iron is not quite the same as in the other metals. Nonetheless, euhedral crystals of any of these minerals may be cubic or, as we will explain in the next chapter, octahedral. More typically, however, these minerals crystallize in less regular shapes. Native zinc, a very rare mineral, has a hexagonal atomic arrangement and so forms crystals of different shapes. The photos below (Figures 9.24 through 9.29) show examples of native gold, silver, copper, platinum, antimony, and sulfur.
Gold, sometimes mined as nuggets or flakes (see the example in Figure 9.17), is also found as wires or scales. Large, visible specimens, like the one seen below in Figure 9.24 are, however, unusual. Most gold and other precious metal ores contain very fine subhedral metal grains, often microscopic. Silver sometimes occurs in a wire-like or arborescent (tree-like) form (Figure 9.25). It also easily tarnishes and so has a gray color in this photo. Most bedrock gold and silver deposits are in quartz-rich hydrothermal veins. Pyrite (fool’s gold) and other sulfides are often associated with native gold and silver in these veins. Besides hard-rock deposits, gold and silver are also found in placers (accumulations in river, stream, or other kinds of sediments), and native silver is found in several other types of deposits. Box 9-3 (below) describes the Witwatersrand gold deposits, the largest gold deposits in the world. Section 9.3.3.1, later in this chapter, gives more detailed information about placers.
Native copper occurs in a variety of ore deposits associated with mafic volcanics and in some sandstones. Copper is found as branching sheets, plates, and wires, and as massive pieces. In Figure 9.26, it is in a discontinuous sheet that has partially altered to malachite, copper carbonate. We mine native platinum primarily from ultramafic igneous rocks, but platinum is also found in placers – Figure 9.27 shows examples placer platinum nuggets. Platinum is also a secondary product of Cu- or Ni-sulfide refining. Native antimony (in Figure 9.28), is rarely pure. It is usually in solution with arsenic and may contain small amounts of other metals. Untarnished specimens are metallic and silvery, but antimony typically tarnishes to a gray color as seen in this photo.
Graphite, diamond, and sulfur are examples of nonmetallic native elements. Figure 9.29 shows an example of native sulfur. Figure 3.49 (Chapter 3) contains another photo. Sulfur deposits are associated with volcanoes, often concentrated at fumaroles. Sulfur is also found in veins in some sulfide deposits and in sedimentary rocks where it is found with halite, anhydrite, gypsum, or calcite. Native sulfur deposits only account for about half the world’s sulfur supply. Most of the rest is separated from sulfides during processing to recover metals.
Both graphite and diamond consist only of carbon. We discussed the nature of the two minerals in Box 3-3 of (Chapter 3). Graphite is common as a minor mineral in many kinds of metamorphic rocks, including marbles, schists, and gneisses. The origin of the carbon is usually organic material in the original sediments. Graphite also occurs in some types of igneous rocks and in meteorites. Diamond only forms at very high pressures associated with the lowermost crust or mantle of Earth. We mine it from kimberlite pipes, where rapidly moving, sometimes explosive, mafic magmas have carried it up to the surface. After formation, diamond sometimes concentrates in river and streambeds where we mine it from placer deposits. Although some diamonds are of gem quality, most are not. We call lower-quality diamonds industrial diamonds or bort (if the diamonds are small and opaque). See section 9.2.4, later in this chapter, for more information about diamonds and other gems.
9.2.2 Sulfides and Sulfosalts
| Sulfide and Sulfosalt Ore Minerals (* = generally metallic) |
|
| sulfides | |
| *pyrite | FeS2 |
| *chalcopyrite | CuFeS2 |
| *molybdenite | MoS2 |
| sphalerite | ZnS |
| *galena | PbS |
| cinnabar | HgS |
| *acanthite | Ag2S |
| *chalcocite | Cu2S |
| *bornite | Cu5FeS4 |
| *pyrrhotite | Fe1-xS |
| *millerite | NiS |
| *pentlandite | (Fe,Ni)9S8 |
| covellite | CuS |
| realgar | AsS |
| orpiment | As2S3 |
| *stibnite | Sb2S3 |
| *marcasite | FeS2 |
| Sulfosalts | |
| *cobaltite | (Co,Fe)AsS |
| *arsenopyrite | FeAsS |
| pyrargyrite | Ag3SbS3 |
| *tetrahedrite | Cu12Sb4S13 |
| enargite | Cu3AsS4 |
Metallic ore deposits contain many different sulfide and related ore minerals. Most are quite rare. The table seen here lists the more important species. Pyrite (iron sulfide) is most common. Other relatively common sulfides include chalcopyrite (copper iron sulfide), molybdenite (molybdenum sulfide), sphalerite (zinc sulfide), galena (lead sulfide), and cinnabar (mercury sulfide). The others in the table are less abundant but are occasionally concentrated in particular deposits.
Sulfide minerals (such as pyrite) contain one or several metallic elements and sulfur as the only nonmetallic element. Bonding is variable: generally either covalent, metallic, or a combination of both. Metallic bonding, too, is important in some species. Other very uncommon minerals grouped with the sulfides (because of similar properties) contain selenium (the selenides), tellurium (the tellurides), or bismuth (the bismuthides) instead of sulfur. A related group of minerals, the sulfosalts, contains the semimetals arsenic and antimony in place of some metal atoms. Because many sulfides have similar atomic arrangements, solid solutions between them are common. The same holds true for the sulfosalts.
Primary sulfide minerals consist of sulfur and reduced metals. When exposed to oxygen rich-groundwaters, or to the atmosphere at Earth’s surface, they easily oxidize or break down in other ways. Oxidation can alter the original mineral’s color or texture. It can also create new minerals. So, iron-bearing sulfides may turn into iron oxide (magnetite or hematite), iron hydroxide (limonite or goethite), or iron carbonate (siderite). Galena (lead sulfide) may become cerussite (lead carbonate). Copper sulfides may become azurite or malachite (both hydrated copper carbonates).
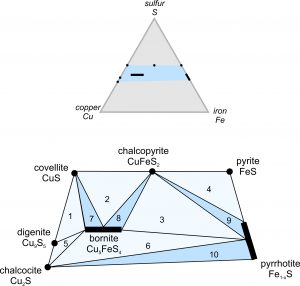
Sulfide minerals often form in common associations. Pyrite, sphalerite, and pyrrhotite are frequently found together, as are chalcopyrite, pyrite, and bornite or pyrrhotite. In some carbonate-hosted deposits, sphalerite and galena occur together. We can depict sulfide associations using triangular composition diagrams. Box 9-4 (below) presents a detailed discussion of Cu-Fe-S ore minerals and explains how we use triangular diagrams to show solid solution compositions.
Unlike other mineral groups, especially the silicates, color is sometimes a good way to identify sulfide minerals, especially for those with metallic lusters (marked with * in the table, above). The reason is that transition metals often control color, and the color of sulfides is often due to the metals they contain. So, color is helpful. Sulfide minerals, however, show lots of variation in appearance, especially if they are tarnished. Space does not permit including photos of all the different sulfides here, but some examples are below.
The most common gold and goldish sulfides are pyrite, chalcopyrite, and pyrrhotite (shown in Figures below). They can be very hard to tell apart. Pyrite, also called fool’s gold, is seen in Figure 9.31. The specimen in the photo has pyrite’s typical metallic golden color. Figures 3.2 and 3.42 (Chapter 3) show other views of golden pyrite. Chalcopyrite, in contrast with pyrite, contains copper and easily tarnishes – often to a yellow-green color. The photo of chalcopyrite below (Figure 9.33) shows multiple colors due to tarnishing. Figures 3.22 and 3.43 (Chapter 3) also show tarnished chalcopyrite. Chalcopyrite is much softer than the other two minerals which also sometimes helps identification. Pyrrhotite, seen in Figure 9.32, is the only one of the three that is magnetic, and sometimes that can distinguish it from the others. Some pyrrhotite has a more silvery color than pyrite which helps identification.
Many sulfides have a gray color, sometimes with metallic luster. The photos below show examples of galena (Figure 9.34), molybdenite (Figure 9.35), and stibnite (Figure 9.36). The lusters of the samples in these photos are not particularly metallic, but many specimens of these minerals are. For example, Figure 3.21 (Chapter 3) shows a spectacular example of metallic stibnite, and Figure 3.40 (Chapter 3) shows a hexagonal flake of metallic molybdenite.
The three photos below show copper minerals. Copper minerals are often characterized by strong colors. Bornite, which is unremarkable and hard to identify if unoxidized, commonly oxidizes and tarnishes to form what we call peacock ore (Figure 9.37). Covellite (Figure 9.38) is usually identified by its blue, commonly metallic color. We include a photo of azurite and malachite here (Figure 9.39) because of the color similarity to the other two minerals. But azurite and malachite are secondary copper carbonate hydroxides and not sulfide minerals. Figure 3.47 (Chapter 3) shows another specimen containing azurite and malachite.
Sphalerite (ZnS) is a mineral that has many different appearances; the three photos below show examples. In Figure 9.40, the sphalerite is dark colored and bordering on metallic. In Figure 9.41, two prominent calcite crystals accompany the sphalerite, which has a characteristic brown resinous appearance. In Figure 9.42, the sphalerite is almost gemmy. Gray galena and orange dolomite are also present in the photo. Because of its many different appearances, sphalerite can be hard to identify unless it is brown and resinous as in Figure 9.41, and in Figure 3.26 (Chapter 3). For example, Figures 3.45 and 3.46 (Chapter 3) showed clear green and yellow sphalerite that looks nothing like the samples seen below. The many different colors of sphalerite are due to trace amounts of iron and other elements in the zinc sulfide. In laboratories, pure manufactured zinc sulfide is white.
The photos below show three of the most colorful sulfides: cinnabar (HgS), realgar (AsS), and orpiment (As2S3). We typically identify these minerals by their color. Cinnabar (Figure 9.43) is generally a red-pink color, although the color is sometime diluted by other minerals present. Realgar (Figure 9.44) has a bright orangey-red color, and orpiment (Figure 9.45) is one of two common minerals (the other is sulfur) that is yellow. Note that the orpiment contains a small amount of orange realgar; their compositions are nearly identical. Figure 3.39 (Chapter 3) contains a photo of orpiment with calcite.
9.2.3 Oxides and Hydroxides
| Oxide and Hydroxide Minerals | |
| oxides | |
| corundum | AlsO3 |
| periclase | MgO |
| magnetite | Fe3O4 |
| hematite | Fe2O3 |
| pyrolusite | MnO2 |
| rutile | TiO2 |
| cassiterite | SnO2 |
| ilmenite | FeTiO3 |
| spinel | MgAl2O4 |
| chromite | FeCr2O4 |
| hydroxides | |
| gibbsite | Al(OH)3 |
| diaspore | AlO(OH) |
| brucite | Mg(OH)2 |
| goethite | FeO(OH) |
| manganite | MnO(OH) |
We often group oxides and hydroxides together because they have similar compositions and atomic arrangements. The table to the left lists the most common of these minerals. These minerals often have similar properties, and most have relatively simple and related formulas. Oxide minerals consist of metal cations bonded to O2-. Hydroxide minerals contain (OH)– anion molecules in place of all or some O2-. We group quartz, the most common oxide, with silicate minerals, so it is not considered here.
A primary difference between oxides and hydroxides is the temperatures at which they form and are stable. Hydroxides are unstable at high temperature; they exist in low-temperature environments and are commonly products of alteration and weathering. Other oxide minerals – magnetite and ilmenite, for example – are high-temperature minerals generally associated with igneous or metamorphic rocks. In fact, most igneous and metamorphic rocks contain oxide minerals. Typically they are present in minor amounts, are easily overlooked, and may be difficult to identify.
Oxides and hydroxides have properties distinct from silicates and sulfides. They are often dominated by ionic bonding, and anions (O2-or OH–) do not control their structure and properties as anions do for other mineral groups. Oxides and hydroxides also are distinct from carbonates, sulfates, and other ionic minerals that often have relatively high solubilities in water.
Simple oxides contain one metal element and have formulas R2O, RO, or R2O3, where R is the metal cation. The different formulas reflect different valences of the metal cations. More complex oxides contain two different metals and have formulas XYO3 or XY2O4, where X and Y denote the metals. Oxides with general formula XY2O4 (spinel and chromite in the table) belong to the spinel group; they all have similar atomic arrangements but contain different elements. Magnetite, which contains both Fe2+ and Fe3+, also has a spinel structure and belongs to this group.
Some oxide minerals, for example corundum and spinel, come in many colors. Figure 3.41 (Chapter 3) shows red ruby and two different colored sapphires (blue and tangerine); all three are corundum. Sapphire may be other colors too, including white, pink, yellow, or orange. The photos below show four different colored spinels that were cut as gems. Pure spinel (which is rare) is clear or light gray like the stone in Figure 9.47 (below left), but most spinel contains trace metals that lead to a wide range of colors. Spinels may be various shades of red, purple, blue, yellow, orange, pink, or black, but red is most common. Some examples are in Figures 9.48, 9.49, and 9.50, below.
Dark colored oxides can be particularly difficult to tell apart, especially because the same mineral can have many different appearances. The photos below shos some typical examples. The top row contains iron oxides/hydroxides. Magnetite (Figure 9.51) is generally silvery or black. It is the only strongly magnetic mineral, which aids identification considerably. Hematite may have a metallic silver color (Figure 9.52) or may be dull to bright red (Figure 9.53) but, in either case, has a red streak. Figure 3.13 (Chapter 3) shows another example of red botryoidal hematite, and Figure 3.48 (Chapter 3) shows hematite’s red streak. Goethite (Figure 9.54), the most common component of rust, may be yellow-brown, black or reddish.
Figure 9.55 shows ilmenite, iron titanium oxide. It sometimes looks like an iron oxide (magnetite or hematite) but is nonmagnetic and has a black streak. The hexagonal flakes of ilmenite in Figure 9.55 are spectacular but rare. Cassiterite, tin oxide, is shown in Figure 9.56. It is harder than the other minerals seen here and often has an adamantine luster. Figure 9.57 is a photo of manganite, a manganese oxide/hydroxide. It shares properties with the other three minerals. The black arborescent mineral in Figure 9.58 is dendritic pyrolusite, manganese oxide, that crystallized along a fracture surface in limestone. Dendritic pyrolusite is sometimes mistaken as having an organic origin. Pyrolusite has other appearances and may be difficult to distinguish from other dense dark-colored minerals. But, it is the only common mineral that forms dendrites like the ones shown.
9.2.4 Gems and Gem Minerals
| Mineral Gems and the Most Important Producing Countries and Regions |
||
| mineral | gem | source |
| beryl | emerald | Colombia, Brazil, Russia, Egypt, East Africa |
| beryl | aquamarine | Brazil, Afghanistan, Pakistan |
| chrysoberyl | alexandrite | Russia, Brazil |
| corundum | ruby | Cambodia, Myanmar, Afghanistan, India, Australia, Thailand, Sri Lanka, Brazil |
| corundum | sapphire | |
| diamond | diamond | Australia, South Africa, Namibia, Russia |
| jadeite | jade | Myanmar, China |
| K-feldspar | moonstone | many sources |
| olivine | peridot | Egypt, Myanmar, Australia |
| opal | opal | Australia, Hungary, Mexico |
| quartz | amethyst | Russia, Sri Lanka, India, Uruguay, Brazil |
| quartz | citrine | |
| topaz | topaz | Brazil, Sri Lanka, Russia, India |
| tourmaline | tourmaline | Namibia, Brazil, United States, Russia |
| turquoise | turquoise | United States, Egypt, Australia |
| tremolite – actinolite | nephrite (jade) | Russia, China, Taiwan, Canada |
| zircon | zircon | Sri Lanka |
Gems are precious or semiprecious stones and related substances that we can cut or polish to use for ornamentation. There are many kinds, and they may be natural or synthetic. Most gems are natural materials; they can be either minerals or nonminerals.
The term gemstone is sometimes used to refer to gems that are minerals. The table lists the common gemstones and their most important countries of origin. Only one or two countries dominate production of many gems, including diamond. Because gems differ in appearances from common minerals, they often have names different from their mineral names. The table contains some examples.
Diamond, emerald (a variety of beryl), and ruby (a variety of corundum), have been, historically, the most valuable gemstones. Sapphire (another variety of corundum) and alexandrite (a variety of chrysoberyl) are nearly as valuable. The most expensive gem on record sold for \$71.2 million during a 2017 auction in Hong Kong. It was a unique oval-shaped diamond with a vivid pink color, weighed about 60 carats, and was almost 2 cm in longest dimension.
It is not the composition of gems that makes them valuable, but rather their appearance. Most are varieties of common minerals that exhibit spectacular color, clarity, brilliance, or play-of-color. The hardest gems – for example diamond, ruby, and sapphire – are especially highly valued because they are most durable. Besides its beauty, a gem’s rarity and uniqueness are important to some people, and gems sometimes have snob appeal.
Common beryl, is opaque with a nondescript blue color. For an example, see Figure 6.89 (Chapter 6). But exceptional beryl crystals are beautiful translucent or transparent gems, including emerald (green), aquamarine (blue), morganite (pink), helidor (yellow), goshenite (clear), and several other varieties. The four photos below (9.59 through 9.62) show gemmy examples of emerald, aquamarine, heliodor, and morganite that could be cut to make colorful gemstones. Although common beryl has no value as a gem, it is sometimes mined as a beryllium ore mineral. Other photos of gemmy beryl were seen in Figures 1.12, 1.13, and 1.14 (Chapter 1).


Some gems, such as the opal shown in Figure 9.63, show fire, a type of play-of-color that appears as changing flashes of different colors when we view a gem from different angles. Fire is most apparent in minerals that exhibit dispersion – the ability to separate white light into different colors that pass through the mineral along slightly different paths. Diamond best exhibits this property (Figure 9.64) but other minerals, including sphene, zircon, and a green garnet called demantoid garnet sometimes also display fire. Fire is most notable in clear gemstones and may be completely masked in strongly colored stones. Fire may also be seen in amber or pearls – which we call gems although they are not minerals. Proper polishing or cutting can enhance play-of-color in gems of many sorts.
We saw other examples of minerals that exhibit play-of-color in previous chapters; all are common gemstones:
•Figures 1.2 (Chapter 1) and Figure 3.52 (Chapter 3) – opal
•Figures 3.51 (Chapter 3) – limonite
•Figures 3.53 (Chapter 3) – labradorite
•Figures 3.54 (Chapter 3) – moonstone

Gems and other minerals derive their color from many different things (see Chapter 3). Common minerals may have little value as gems, but if we can alter or enhance colors, even common quartz may become valuable. Gemologists, therefore, often treat gems and minerals, natural or synthetic, to change or enhance their color and increase their value. The vast majority of gems on the market today have had their appearances enhanced in some way.
For instance, quartz crystals from the Hot Springs area of Arkansas are irradiated to disrupt their atomic structure and give them a smoky, purple, or black color. Irradiation is also used to induce color changes in diamond and topaz. Figure 9.65 shows a remarkable blue colored topaz crystal. The strong color was produced by irradiation.
Gemologists also change a stone’s color using dyeing or heat treatment, although dyeing only affects gems such as jade and chalcedony, that are porous. Gemologists have successfully used heat treatment – which changes elemental valences or alters atomic structures – on quartz, beryl, zircon, and topaz, although the results are not always predictable.
9.2.4.1 Synthetic Gems

Today, it is routine to synthesize gems of many sorts, including diamonds, and also varieties of quartz, beryl, corundum, and garnet. Chrysoberyl, opal, rutile, spinel, topaz, and turquoise are also synthesized. We saw photos of both natural and synthetic topaz and ruby in Figures 1.16, 1.17, 1.18, and 1.19 (Chapter 1). The photos seen here in Figure 9.66 show synthetic sapphire, ruby, and emerald.

Several synthetic compounds with no natural equivalents are used as simulants for gems. Foremost among them are yttrium aluminum garnet (YAG; Y3A15O12) and cubic zirconia (CZ; ZrO2), both used as imitation (or “genuine faux” from the French for “fake”) diamonds. This photo (Figure 9.67) shows different examples of (synthetic) cubic zirconia. Today, clear CZ – two examples are seen in the photo – is the most common diamond simulant. CZ can have just about any color and so is a common simulant for other, darker-colored, gems as well.
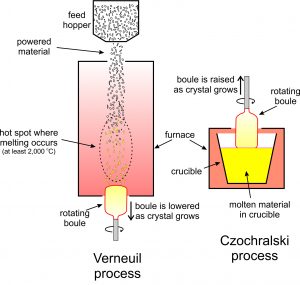
Gem manufacturers use several different methods to produce synthetic crystals. The Verneuil process and the Czochralski process both involve crystallizing gems from molten material (Figure 9.68). In the more common Verneuil process, powdered starting materials pass through a hot furnace and melt to produce droplets that add to a growing boule, a single elongated crystal, at the bottom of the furnace. The boule is slowly withdrawn from the furnace as it grows. This technique produces large synthetic rubies, sapphires, spinels, and other gems. The synthetic rubies are often key components of lasers.
In the Czochralski process (also shown in Figure 9.68), a seed crystal is placed in contact with a melt and allowed to grow. As it grows, the crystal is raised from the melt and so grows even longer. The photo on the left, below in Figure 9.69, shows synthetic corundum, including red ruby, made using the Verneuil process. The other colored stones are generically called sapphire. The stones in the bottom of the photo have been faceted to be used as ornamental gems. The photo on the right, in Figure 9.70, shows ruby being synthesized using the Czochralski process. The Czochralski process can produce 40 cm, or longer, ruby boules.
A third approach, the flux method, has sometimes yielded large synthetic crystals, notably ruby, sapphire, alexandrite, and emerald. A flux is a material that promotes reaction and crystal growth but is not incorporated in crystal structures. A mixture of chemicals – including those needed to make the desired mineral and others to act as the flux – is ground together and heated above its melting point. As the temperature is lowered, crystals begin to grow. After the melt solidifies, water or other reagents remove the flux, leaving the desired crystals. Most synthetic rubies and emeralds are created this way, but the process is very slow and may take many months. A related approach, called the hydrothermal method produces synthetic quartz and a few other gems. The method involves heating water that contains the necessary dissolved elements, and letting the crystals grow as the solution cools. It is a very slow process, like the flux method.
Mineralogists synthesize minerals in other ways, but they rarely produce gems of great value. For example, synthetic minerals may be grown from hydrothermal solutions in high-pressure reactor vessels called bombs. Synthetic quartz, corundum, and emerald have all been made this way. Synthetic diamond and a few other high-pressure minerals are made using a solid state (no melt or water) approach. This process involves a cylinder of starting material, enclosed in a graphite heater, squeezed between two pistons. Electricity passing through the graphite heats the material to temperatures at which crystals will grow. Chemists at General Electric have perfected this technique for making gem-quality synthetic diamonds.
9.2.4.2 Cutting and Polishing Gems
Most gems have shapes that do not resemble natural crystals. Sapphire, a variety of corundum, generally grows as hexagonal crystals, but when sapphires are to be incorporated into jewelry, gem cutters shape and polish them to increase their beauty and value. Figure 9.71 below shows natural sapphire from Madagascar and Figure 9.72 shows the Logan sapphire, a famous gemstone originally from Sri Lanka. Twenty diamonds surround the sapphire. Gem cutting affects value as much as the quality of the raw material. Cutting and polishing takes place in many parts of the world. Israel and Belgium dominate diamond cutting; the United States, India, Hong Kong, Thailand, and Brazil also cut significant amounts of various gems.

Gemologists shape gems in several ways. Agate, opal, chalcedony, and onyx may be tumbled in a cylinder with a polishing/abrading agent. The cylinder rotates until the stones have smooth surfaces. The stones become polished, but often have irregular shapes. Alternatively, gemologists shape stones using a wet grinding wheel made of quartz sandstone (for relatively soft minerals) or metal impregnated with diamonds (for harder minerals). After shaping and polishing, the stones are called cabochons. They have a smooth, domed top and, usually, a flat base. Until the Middle Ages, most gems were cut as cabochons. The gems shown in Figure 9.73 are all varieties of the mineral tourmaline, shaped and polished as cabochons.

While most soft gemstones receive a cabochon cut, many hard gemstones are faceted. Facets are small, polished, planar surfaces that give the stones attractive shapes and light properties. With proper cutting, originally dull stones can sparkle. Facets are usually symmetrically arranged in geometric shapes. Gemologists create them by mounting stones on a holder, called a dop, and grinding the stone with a diamond-impregnated wheel. As with cabochons, the gems are polished after being ground. Figure 9.74 shows a faceted example of orthoclase from Madagascar. Figures 9.47-9.50 (spinel), 9.64 (diamond), 9.66 (synthetic sapphire, ruby, and emerald), 9.67 (cubic zieconia), and 9.69 (synthetic ruby and sapphire) contain other photos of faceted gems.
9.3 Types of Ore Deposits
9.3.1 Magmatic Ore Deposits

Minerals containing elements of economic value are generally present in all igneous rocks, but the elements may not be concentrated enough to make mining economical. Only in relatively rare circumstances are they in sufficient abundance so that mining is profitable. If the minerals are scattered throughout a host rock, but in sufficient amounts to mine profitably, we call the deposit a disseminated deposit. Disseminated deposits produce most of the world’s diamonds, copper, and molybdenum and also large percentages of the available tin, silver, and mercury. Often, disseminated ores consist of minerals scattered randomly in a host rock.
Sometimes geological processes concentrate ore minerals in vein deposits consisting of veins that are centimeters to meters thick. If ore is distributed in many small veins, geologists call the deposit a lode deposit. Vein deposits account for most of the world’s gold and silver mines, and also some copper and lead-zinc mines. Figure 9.75 shows a meter wide quartz vein that is the source of gold at Nalunaq Gold Mine in southern Greenland. In still other kinds of igneous deposits, ore minerals become concentrated in layers. Below, we look at some of the most important kinds of igneous deposits.
9.3.1.1 Magmatic Sulfides and Cumulates
Mafic and ultramafic magmas, like all common magmas, contain the major elements oxygen, silicon, aluminum, iron, calcium, sodium, potassium, and magnesium. But they typically also contain other elements including sulfur, nickel, and less common metals such as platinum, palladium, and chromium. As these magmas cool and crystallize, the first minerals to form are plagioclase, pyroxene, and olivine – all made of major elements. Consequently, the concentrations of sulfur and other minor elements increase in remaining melt. Eventually, sulfur concentration becomes great enough so that sulfide minerals begin to crystallize. The sulfide minerals, typically containing iron and nickel, may also contain relatively high concentrations of platinum, palladium, and other minor metals.
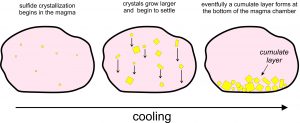
Sulfides have greater densities than silicate minerals and the mafic or ultramafic melts. So, the denser sulfide minerals will, over time, begin to sink, as shown in Figure 9.76 above. Eventually, after more cooling and crystallization, significant deposits of sulfide minerals may accumulate on the bottom of a magma chamber. The deposits, which may form centimeters-, or meters-thick layer called a cumulate, are often entirely, or nearly entirely, composed of sulfide minerals. This process produces magmatic sulfide deposits, which are the most important sources of platinum, palladium, chromium, and several other metals. Cumulate sulfide minerals include pentlandite (Fe,Ni)9S8, chalcopyrite (CuFeS2), pyrrhotite (Fe1-xS), and pyrite (FeS2).

Cumulate sulfide deposits account for almost 60% of the world’s nickel production and more than 95% of platinum and palladium production. These deposits are associated with mafic and ultramafic magmas but not, generally, with felsic magmas, because felsic magmas are so viscous that they cool and crystallize before dense minerals can settle.
Sulfides are not the only kind of mineral that can become concentrated in a cumulate deposit. Oxides – including magnetite (Fe3O4), ilmenite (FeTiO3), and chromite (FeCr2O4) – may settle and collect at the bottom of a magma chamber, too. South Africa’s Bushveld Complex is the most significant and important example of oxide mineral cumulates. Figure 9.77 shows Bushveld chromite layers surrounded by a feldspar-rich rock. These chromite cumulates produce not only significant amounts of chrome, but also very large amounts of platinum, palladium, and related elements.
9.3.1.2 Pegmatites

During crystallization, some minerals crystallize before others. Consequently, a late-stage magma will not be the same composition as an original magma. Pegmatite is the name given to coarse-grained igneous rocks that form during the final stage of magma crystallization. Common pegmatites have an overall granitic composition and comprise mostly quartz, feldspar, and mica. But pegmatites share another important characteristic. They also commonly contain minerals made of relatively rare elements that did not go into the early formed minerals. Figure 9.78, for example, contains the common minerals K-feldspar (white) and quartz (gray), but also contains tourmaline (black), a boron mineral. (Biotite is also present but is hard to see in the photo.) So, pegmatites are often mined for minerals rich in boron, cesium, lithium, molybdenum, niobium, tantalum, tin, tungsten, or other elements. For example, pegmatites are sometimes sources of spodumene (an important lithium mineral) and beryl (an important beryllium ore mineral).
Additionally, pegmatites may be a source of gem minerals. Gems from pegmatites include emerald and aquamarine (varieties of the mineral beryl), amazonite (variety of feldspar), apatite, chrysoberyl, garnet, spodumene, lepidolite, topaz, tourmaline, zircon, and others. Most pegmatite mines make their money from mineral specimens and gems rather than from the ore minerals they contain. The minerals in Figures 9.59 – 9.62, earlier in this chapter came from pegmatites, and we saw other examples of pegmatite minerals in Chapter 4 and Chapter 6:
• Figure 4.1 – Aquamarine and tourmaline
• Figure 4.12 – Riebeckite (an amphibole) with K-feldspar and quartz
• Figure 4.13 – Tourmaline
• Figure 4.30 – Emerald (beryl) on quartz
• Figure 6.108 – Tourmaline from a pegmatite
9.3.1.3 Kimberlites

Kimberlites, named after the town of Kimberly, South Africa, where they were first described, are volcanic rocks that originate in Earth’s mantle. They are mined exclusively for diamonds. The photo in Figure 9.79 shows the “Big Hole” at Kimberly. The Hole was mined from 1871 to 1914 and reached a depth of 240 m below the surface. Subsequently it filled with water.
Kimberlite eruptions are gas-powered explosive events. The magmas originate at depths of 150 to 450 km, deeper than other igneous rocks. Most kimberlites are in small vertical columns called kimberlite pipes although some rare sills are known. These pipes are the most important source of diamonds today. If kimberlite weathers and erodes, the diamonds may become concentrated in sedimentary deposits. Some kimberlites bring mantle xenoliths (pieces of mantle rock) to the surface; petrologists use these samples to study mantle chemistry and mineralogy.
Kimberlites are ultramafic rocks, having high magnesium and low silicon contents, and are rich in potassium. Mg-rich olivine and carbonate minerals generally dominate, but some kimberlites contain significant amounts of phlogopite (Mg-rich biotite). Lesser amounts of serpentine, ilmenite, garnet, clinopyroxene, enstatite and chromite may be present. Most kimberlites are very old, having erupted between about 80 million and 2.5 billion years ago. A few younger ones, 10-20,000 years old, are in Tanzania and Democratic Republic of the Congo.
Figure 8.11 (Chapter 8) showed a kimberlite that contains diamonds. Not all kimberlites do. The photo below in Figure 9.80 shows a sample of kimberlite from Baffin Island, Canada. The dark material consists of pyroxene and olivine. The obvious bright green minerals are chrome diopside. Harder-to-see smaller wine-red minerals are Mg-rich garnet. The large light-colored fragments are limestone that got caught up in the kimberlite magma during eruption.

9.3.2 Hydrothermal Ore Deposits
As a melt cools and crystallizes, hot, water-rich fluids may be released. These hydrothermal fluids are rich in sulfur, sodium, potassium, copper, tin, tungsten, and other elements with relatively high solubilities. Hydrothermal fluids dissolve other elements as they flow through rocks and eventually cool to deposit minerals in hydrothermal deposits. These deposits fall into four or five categories: porphyry deposits, skarn deposits, volcanogenic massive sulfide deposits, sedimentary exhalitive deposits, and epigenetic deposits. We discuss the different types in sections below – they have distinctly different origins and vary in size from huge networks of veins covering many square kilometers to small veinlets only centimeters wide. Hydrothermal deposits generally form at mid-ocean ridges, in subduction zone, or next to plutons. In all these settings, there is a source of heat that drives fluid circulation. The exceptions are epigenetic deposits that may form in continental interiors.
The Nalunaq gold shown in Figure 9.16 is an example of hydrothermal gold. Two other examples of hydrothermal mineralization are below in Figures 9.81 and 9.82: molybdenite ore from the Keystone Mine in Colorado and gold ore from the Sierra Nevada Mountains.
Many minerals trap droplets of hydrothermal fluids that we can analyze to get information about their origins (Box 9-5). But, hydrothermal fluids may travel long distances before they deposit ore. So, determining the source of the fluid and of the ore elements is often difficult or impossible. Explaining deposition, however, is easier. It usually occurs in response to cooling, pressure changes, or changes in pH or other chemical factors.
9.3.2.1 Porphyry Deposits

Porphyry deposits are a special kind of hydrothermal deposit. They form when hydrothermal fluids, derived from magmas at depth, carry metals toward the surface and deposit minerals to create disseminated ore deposits. These deposits are important sources of copper, molybdenum, and gold. They may also yield tungsten or tin.
In porphyry deposits, ore minerals are in small veins within a hydrothermally altered host rock, generally a porphyritic felsic to intermediate composition intrusive rock. Porphyry ores are not particularly high-grade, but the deposits are commonly large and so are profitably mined. These deposits are common in the mountains along the west coast of North and South America, and in islands of the southwest Pacific, north of Australia.
The Morenci deposit in Arizona is a porphyry deposit. Figure 9.84 shows a satellite image of the mine. The town of Morenci (pop 1,489) is in the lower right of the photo. Copper minerals were first discovered at Morenci by an Army battalion in 1865; mining began in 1872. Today, the mine, with pits that total almost 130 square kilometers, is the largest copper producer in North America. The workings extend beneath and between several large mountains next to the Morenci town site. Pyrite and chalcopyrite, both sulfide minerals, are the primary copper ore minerals, but chrysocolla (copper oxide/hydroxide) and malachite (copper carbonate) are found and mined from oxidized ore zones. Although copper minerals are by far the most important ore minerals, the mine also produces lesser amounts of sphalerite (zinc ore), galena (lead ore), and molybdenite (molybdenum ore).
9.3.2.2 Skarn Deposits

As described in Chapter 8, skarns are contact metamorphic zones that develop around an intrusion. They may be thin or thick, and their formation often involves metasomatism. Skarns can form in any kind of rock, but most are associated with limestone or dolostone. Common skarn minerals include calcite and dolomite, and many Ca-, Mg-, and Ca-Mg-silicates. Some skarns, however, are valuable mineral deposits containing copper, tungsten, iron, tin, molybdenum, zinc, lead, and gold. Skarns account for nearly three quarters of the world’s tungsten production. Less commonly, skarns produce manganese, nickel, uranium, silver, boron, fluorine, and rare-earth elements. Porphyry deposits and skarn deposits are both the results of hydrothermal activity, and a continuum exists between the two types. The photo in Figure 9.85 shows underground mining in a major skarn that yields tungsten, Canada’s Cantung Mine in the Northwest Territories.
9.3.2.3 Volcanogenic Massive Sulfides and other Exhalitive Deposits

When hydrothermal fluids create ore deposits at, or near, Earth’s surface, we call the deposits exhalitives. The Kidd Mine, shown in Figure 9.86, is an example. The mine is in eastern Ontario, near Timmins; it is the world’s deepest base metal (a term referring to industrial metals excluding iron and precious metals) mine and extends to a depth of more than 3,350 meters. The bottom of the mine is said to be the closest a person can get to the center of Earth.
The Kidd Mine started producing in 1966. It was initially an open pit but soon went underground. The moneymaking metals are mostly copper and zinc, but silver, gold, lead, and other metals are important too. Ore minerals – mostly pyrite, pyrrhotite, chalcopyrite, sphalerite, and galena – were deposited when warm, metal-rich hydrothermal waters combined with ocean waters in sediments and rocks of the ocean floor. The resulting deposits are in pods or sheets within sedimentary rock layers that, in places, contain nearly 100% ore.
The Kidd ore deposit is an example of a volcanogenic massive sulfide (VMS) deposit. Most such deposits are small, and, although the Kidd Mine is large, it is not the largest. The largest VMS deposits, about twice the size of the Kidd, are the Windy Craggy deposit in British Columbia, discovered in 1958, and the Rio Tinto deposit in Spain, discovered in 1972. The quality of the ore in massive sulfide deposits is high, host rocks are generally greater than 60% ore minerals, so even if they are small, massive sulfide deposits are alluring mining prospects. The photo below in Figure 9.87 shows one of the main pits at the Rio Tinto Mine. The name of the mine translates to Red River, and the photo in Figure 9.88 shows the acid mine drainage that gives the river its color today. Runoff from the mine has caused major environmental problems.
 Click this link to see a 4 minute YouTube video of a black smoker near the Galapagos Islands. |
What makes VMS deposits especially intriguing is that we can see them being created today. This photo (Figure 9.89), and the spectacular YouTube video that is linked below it, show a black smoker on the ocean floor. At these smokers, hot hydrothermal waters, mixing with ocean waters, create fine particles of sulfide minerals and produce massive ore deposits. The iron sulfides that are the most common minerals created, are black, so the name. The ores mined from the Kidd, Windy, Craggy, Rio Tinto, and other massive sulfide deposits owe their origins to black smokers such as the one seen here. The smokers cover huge regions of the ocean floor and did so in the past. After forming, they later became uplifted and incorporated into the continents where we find them today. As seen in the video, black smokers are also sites of abundant marine life.
Black smokers occur at all mid-ocean ridges and mid-ocean ridge ores are potentially minable. Prospecting of ocean floors is occurring today, and some mining companies have developed tentative plans for mining operations. To date, however, the water depth has proven too great for direct mining. Some areas near black smokers contain sulfide ooze that might, perhaps, be picked up with a vacuum. The Papua New Guinea (PNG) government invested more than \$100 million in the Solwara 1 project, a planned mining operation that was to target mineral-rich hydrothermal vents on the ocean floor just north of PNG. The project had significant funding problems and met with much local and environmental opposition. It was cancelled in 2019.

Sedimentary exhalitive (SEDEX) deposits are close cousins to VMS deposits. The difference is that host rocks in SEDEX deposits are sedimentary rocks. These deposits are rare compared with the other deposit types already discussed. They have produced significant amounts of zinc, lead, silver and sometimes copper. But, most of them are not economical to mine. Figure 9.90 shows copper ore (mostly chalcopyrite and bornite) from the Rammelsberg SEDEX deposit in Germany. At Rammelsberg, the hydrothermal ores are in shale. The Rammelsberg mine once produced silver, copper, and lead but is closed today.
9.3.2.4 Epigenetic Deposits
When a hydrothermal deposit is not directly associated with a pluton, we call it an epigenetic deposit. Often, the hydrothermal fluids have traveled so far that their original source is unknown. For example, some flat sedimentary rocks in the interior of the United States have strata of limestone that contain ore minerals. These include mineral deposits of the Southeast Missouri Lead District and related deposits in Iowa, Wisconsin, and Illinois. The deposits are especially concentrated in a curved zone called the Viburnum Trend in southeast Missouri. Similar deposits are found at Pine Point in Canada’s Northwest Territories, in northern, England, and in a handful of other places around the world. We call all these deposits Mississippi Valley type (MVT) deposits.

This photo (Figure 9.91) shows a museum specimen from an MVT ore deposit in the North Pennines of England. This sample is mostly green fluorite, but also contains silver-gray cubes of galena and white and salmon-colored calcite.
Primary ore minerals in MVT deposits are generally galena (PbS) and sphalerite (ZnS). Fluorite (CaF2) is common but has little economic value. Weathered or altered MVT ores may contain anglesite (PbSO4), cerussite (PbCO3), smithsonite (ZnCO3), hydrozincite (also a type of zinc carbonate), and secondary galena or sphalerite. Flowing groundwaters deposited both primary and secondary ore minerals long after limestone formation, but the origins of the groundwaters are unknown. According to some geologists, the metal-rich ore fluid came from oxidized clastic iron-rich rocks.
9.3.3 Sedimentary Ore Deposits
9.3.3.1 Placer Deposits
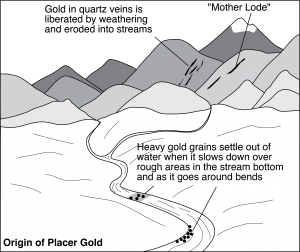
Gravity may be an important force that concentrates economic minerals. Heavy minerals, weathered from igneous, sedimentary, or metamorphic rocks, can be picked up and rivers may transport them long distances before they become concentrated in placers. So, placer deposits, also just called placers, form when one or more minerals concentrate in this way to become an ore deposit. The word placer is Spanish for alluvial sand.
Typically, placers form where a stream’s velocity slows on point bars, in braided streams, or in alluvial fans (Figure 9.92). Other similar deposits are in beach sands or gravels on ocean and lake shores. And some placers, although not commonly mined, form in offshore marine environments on continental shelves.

Placer gold set off the historically important California Gold Rush of 1849 (Figure 9.93). The original sources of minerals in placers are often difficult to determine but, in California, the source has been identified. The California gold weathered from extensive vein deposits, called the mother lode, in the Sierra Nevada Mountains. The lode is in a zone east of Sacramento and San Francisco that is 1 to 6 km wide and extends almost 200 km north-south. Mother Lode gold is in quartz veins up to 20 meters thick and thousands of meters long. Many prospectors told stories of finding and mining the mother lode, but in actuality, most of it eroded away long before the miners arrived.
The California gold rush resulted in California being admitted to the United States in 1850 and later provided a name for a San Francisco football team (the 49ers). Although once one of the most productive gold-producing districts in the United States, the Mother Lode is presently mostly a tourist destination and the home of wineries.

Placer minerals must be both dense and durable to be deposited and remain in place without decomposing. Native metals such as copper or gold, sulfide minerals such as pyrite or pyrrhotite, and oxide minerals such as magnetite or ilmenite are all dense and likely to be found in placers. Metal oxides, especially magnetite (iron oxide), are common and especially dense and durable, and often dominate such deposits. And gold is dense and extremely resistant to any kind of weathering and so can accumulate in stream and river sediments. Gold in placer deposits is found as nuggets that range from microscopic size to basketball size. The photo seen here (Figure 9.94) shows millimeter and finer-sized placer gold nuggets from an unknown origin.
Other important metals besides gold come from placers. As discussed previously, during the Bronze Age, people of Mesopotamia and Greece obtained their tin from what is today England. That tin came from tin placers in the modern-day area of Cornwall. The tin is in cassiterite (SnO2) that derived from the weathering of nearby granites. People mined the Cornwall tin deposits, placer and underground, for more than 4,100 years, beginning about 2150 BCE. The last mine, the South Crofty Mine, closed in 1998. Other important minerals found in placers include diamond, garnet, ilmenite and rutile (titanium ore), ruby, sapphire, monazite, and zircon.
9.3.3.2 Iron Ores

Sedimentary ore deposits also form by chemical precipitation; banded iron formations (BIF), found in Precambrian shields, are examples. Photos of BIF are seen here in Figure 9.95, and also in Figure 7.77 (Chapter 7). Banded iron formations are massive in scale, in places covering hundreds of square kilometers, and perhaps being tens to hundreds of meters thick. If they contain especially significant amounts of magnetite and hematite, they are profitably mined.
Typical banded iron formation contains repeating layers of black to silver iron oxide (magnetite), and red chert (microcrystalline quartz). The overall red color is because the chert contains inclusions of hematite. The outcrop shown in Figure 9.95 is in the Mesabi Iron Range of Minnesota. It formed during the Precambrian Eon, 2.1 billion years ago, and is 2 meters high, 3 meters across, and weighs 8.5 tons.
Banded iron formations include oxides, silicates, and carbonates of iron. They are most commonly rich in magnetite (Fe3O4) and hematite (Fe2O3) but siderite (FeCO3), and the iron hydroxides goethite and limonite are sometimes ore minerals. Other minerals in BIF include the carbonate ankerite (similar to siderite with impurities), and iron silicates such as minnesotaite (an iron amphibole), greenalite (Fe-rich serpentine), or grunerite (also an iron amphibole). Changes in the composition of Earth’s atmosphere more than two billion years ago caused deposition of these minerals. They are commonly associated with very old fossil algae which likely caused the atmospheric change. BIFs are found on all the world’s major continents, and, where they are found, mining often occurs.

Australia contains many large iron mines where hematite is the main ore mineral. Most large hematite deposits formed by alteration of banded iron formations. These deposits are less common than magnetite-rich banded iron formation but easier to mine and process.
Australia dominates the world iron market, producing 36% of all iron ore. Brazil and China each produce about 16%. Figure 9.96 shows an iron mine at Tom Price in Western Australia. The bedrock and the soil have the typical reddish color associated with oxidized iron minerals, such as hematite and goethite, that typifies iron formations.
9.3.3.3 Evaporites
When a body of water is trapped, evaporation can lead to precipitation of halite and other salts. Thick evaporite deposits of halite, sylvite, gypsum, and sulfur have formed in this way. Section 7.3.2 (Chapter 7) discussed the formation of these deposits and evaporite minerals. Evaporites are mined for many things, most notably halite, sylvite, and gypsum. They also produce boron- and lithium-mineral ores.
9.3.3.4 Laterite Deposits
The weathering of preexisting rock may expose and concentrate valuable minerals. Over time, water leaches rocks and soils, dissolving and carrying away soluble material. The remains, called residuum, may be rich in aluminum, nickel, iron, or other insoluble elements. In tropical climates extreme leaching has produced soils called laterites, which are rich in aluminum or, sometimes, nickel. If aluminum-rich laterite lithifies to become rock, we term it bauxite . We mine laterites and bauxites from open pits to produce nickel and aluminum and, sometimes as a secondary commodity, iron. Chapter 7 discussed the formation of laterites and bauxites. See especially Box 7.1.
9.3.3.5 Phosphorites

Phosphorus, like potassium, is an essential nutrient for all living things and people have used it as a fertilizer for centuries. In the 18th century, people used bone ash as a source of phosphorus, created by roasting the bones of animals to liberate contained phosphorus. In the 19th century, people turned to guano, or bird and bat excrement, as the principal source of phosphorus for fertilizer. They obtained guano from small ocean islands with large bird populations. Figure 9.97 shows a typical island with white guano deposits. Similar deposits are found on many ocean islands around the world, where tens of thousands to millions of years of bird excrement has collected. In some places, layers of guano are up to tens of meters thick.
Today, instead of coming from bird dung, most phosphorus comes from phosphorite – which is what is being mined in the photo below in Figure 9.98 – a phosphate-rich chemical sedimentary rock that forms in several different marine environments. The most common depositional environments are in shallow, near-shore marine settings, including beaches, intertidal zones, and estuaries. The mine in this figure is in Togo, West Africa.
Figure 9.99 shows the Bou Craa phosphorite mine in the interior of Western Sahara (a disputed territory south of Morocco). A 60-mile long covered conveyor belt carries ore from the mine to a shipping port on the Atlantic Coast where it is transported for use a fertilizer around the world. Morocco and Western Sahara contain 70% of the world’s known phosphate deposits today, but China is the number one producer. Some smaller, but still significant production occurs in other countries. In the United States, we sometimes mine phosphorite in Florida, Idaho, North Carolina, and Utah.
|
|
|
white line
white line
●Figure CreditsUncredited graphics/photos came from the authors and other primary contributors to this book. 9.1 Pyrite with hematite, Didier Descouens, Wikimedia Commons |