8 Metamorphic Minerals and Metamorphic Rocks
KEY CONCEPTS
- Metamorphic minerals and rocks form when original parent rocks (protoliths) undergo changes in chemistry, texture, or composition.
- Heat and pressure are the most important causes of metamorphism. Metamorphic fluids flowing through a rock may cause significant changes in some cases.
- Different metamorphic textures characterized different kinds of metamorphic rocks.
- Chemical reactions of many sorts occur during metamorphism.
- Different parent-rock compositions produce different kinds of metamorphic rocks.
- The composition of the parent rock determines the metamorphic minerals and rocks that may form.
8.1 Different Kinds of Metamorphism
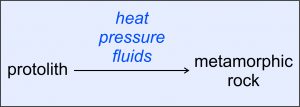
Metamorphic rocks, and the processes that create them, are key parts of the rock cycle that relates igneous, sedimentary, and metamorphic rocks. Most metamorphic rocks form when heat, pressure, or chemically reactive fluids cause changes in preexisting rocks (Figure 8.2). The preexisting, or parent rocks, are called protoliths. Protoliths can be igneous, sedimentary, or metamorphic rock of all sorts. The changes that occur during metamorphism may involve changes in rock texture, in the minerals present, and sometimes in overall rock composition. These changes record geologic processes and events of the past. Consequently, metamorphic petrologists often study metamorphic rocks to interpret rock histories.
8.1.1 Regional Metamorphism
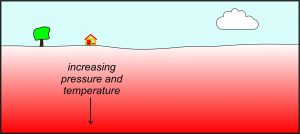
The most significant causes of metamorphism are mountain building processes (tectonism) that bury, while heating and squeezing, rocks. This kind of metamorphism, called regional metamorphism, creates large metamorphic terranes, regions characterized by distinctive metamorphic rocks and intensity of metamorphism that may vary laterally. Regional metamorphism occurs because both pressure and temperature increase with depth in Earth (Figure 8.3). The deeper the rocks, the greater the metamorphism. The photos below show two outcrops of regional metamorphic rocks. The schist outcrop on the left (Figure 8.4) is in Vermont’s Green Mountains; it formed about 450 million years ago during metamorphism associated with the Taconic Orogeny. The gneiss seen in outcrop on the right (Figure 8.5) is much older; it is from Precambrian terrane 750 km northwest of the Green Mountains, near Sudbury, Ontario.
Mountain building brings rocks from deep in Earth to the surface. So, many examples of regional metamorphism are found in mountain belts, for example the outcrop in Green Mountains in Figure 8.4, above. Other examples are found in Precambrian shields, relatively flat-lying areas that may be thousands of kilometers across, that are the exposed roots of ancient mountains. The gneiss seen in Figure 8.5 is from the Canadian Shield in central Ontario.
8.1.2 Contact Metamorphism
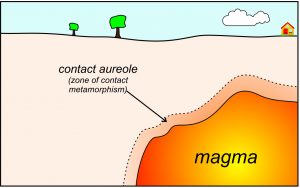
Although regional metamorphism, which accounts for most metamorphism, generally occurs at relatively deep levels within Earth, metamorphism can also occur at shallow levels or even at Earth’s surface. This occurs when magma that intrudes the crust rises close to, or all the way to, the surface. In such cases, heat from the magma can cause contact metamorphism that affects shallow or surface rocks. The effects of contact metamorphism may be profound because of the high temperature contrast between magma temperatures and upper crustal rock temperatures.
As seen in Figure 8.6, contact metamorphism leads to the development of metamorphic zones called contact aureoles, or skarns, that wrap around an intrusion. Aureoles may be anywhere from a few centimeters to many kilometers thick. The formation of contact aureoles frequently involves metasomatism, a change in rock composition due to flowing metamorphic fluids. The width of an aureole mainly depends on the size of the intrusion and how much fluid (mostly H2O and CO2) it gives off. Aureoles often develop concentric zones or layers, each containing distinct metamorphic minerals and mineral assemblages that reflect the maximum metamorphic temperature attained and the amount of metasomatism. These zones, too, vary from being quite thin to being kilometers thick.
8.1.3 Hydrothermal Metamorphism
Occasionally metamorphism occurs without significant tectonism or magmatism. For example, metamorphism called hydrothermal metamorphism may occur because of hot water flowing through rock in areas next to hot springs or other geothermal areas. And, sometimes, water flowing through vast regions of the crust alters rocks far from mountain belts. These changes, mostly chemical in nature, can occur without significant increases in temperature and pressure. Typically, however, hydrothermal metamorphism is associated with regional or contact metamorphism.
8.1.4 Dynamic Metamorphism

Metamorphism can also occur when rocks grind together, producing dynamic metamorphism, also called cataclastic metamorphism. This uncommon form of metamorphism, occurs because of shearing and deformation associated with faults and fault zones where rocks move past each other. The metamorphism produces fractured and granulated rocks that contain elongated mineral grains. Sometimes, but not often, new metamorphic minerals form. Figure 8.7 shows an outcrop where dynamic metamorphism has occurred along a meter-wide fault zone.
8.1.5 Impact Metamorphism

Impact metamorphism, also called shock metamorphism, is related to dynamic metamorphism because it also involves physical changes involving crushing and deformation. This kind of metamorphism occurs when a meteorite impacts Earth. The products may include high-pressure metamorphic minerals such as coesite or stishovite, both polymorphs of quartz. Highly granulated, deformed, and shattered rocks are common, and sometimes intriguing structures called shatter cones develop. Figure 8.8 is a photo of shatter cones created by a meteorite impact in Quebec. Shatter cones are akin to the damage that a pebble does when it strikes the windshield of your car.
Regional and contact metamorphism account for most metamorphic rocks. Dynamic and impact metamorphism are in distant third and fourth places. Some geologists have also described another kind of metamorphism, called burial metamorphism, but it is really just high-temperature diagenesis.
8.1.6 Rocks of Different Metamorphic Grade
Metamorphic grade is a general term we use to describe the temperature at which metamorphism occurs. Metamorphic grade is important, not just because different kinds of rocks and minerals form at different temperatures, but because temperature affects chemical reaction rates. Rocks metamorphosed at low temperature may change only very slowly, and some changes may not go to completion. Rocks that form at high temperatures generally do not have the same problems. However, there are many kinds of metamorphic rocks, and some of them are more chemically reactive than others.

Low-grade metamorphic rocks form at low temperatures, generally between 150 and 450 °C. They mostly form at low pressures, too. At the lower end of this range, diagenesis overlaps metamorphism. Low-grade metamorphic rocks are often fine grained. Because they are hard to study and frequently do not represent chemical equilibrium, many metamorphic petrologists prefer to study higher-grade rocks. The photo in Figure 8.9 shows a serpentinite, an example of a low-grade metamorphic rock. It contains serpentine and chlorite, both hydrous minerals, that formed during metamorphism of a mafic protolith.
Medium-grade metamorphism, forming at temperatures between 400 and about 600 °C, often produces rocks containing conspicuous metamorphic minerals we can easily see and study. Many schists are medium-grade rocks.

High-grade metamorphic rocks, which form at temperatures greater than about 600 °C, are usually quite coarse-grained and contain minerals easily identified in hand specimen. Most form at high pressures. The high-grade rock shown in Figure 8.10 contains conspicuous centimeter-sized red garnet, black hornblende, and white plagioclase feldspar.
Depending on its composition, a high-grade metamorphic rock may undergo partial melting, also called anatexis, so both metamorphic and igneous processes contribute to its evolution. When this happens, the rock, strictly speaking, is no longer a metamorphic rock. We call the resulting partially melted rocks migmatites, which means “mixed rocks.” For some composition rocks, partial melting may begin at temperatures as low as 700 °C. Other kinds of rocks, especially those that contain little H2O, may remain completely solid to temperatures as great as 1100 °C.
8.1.7 Prograde and Retrograde Metamorphism
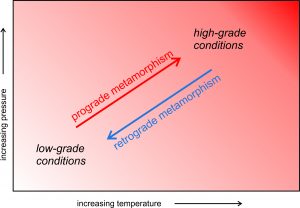
Prograde metamorphism occurs when low-grade or unmetamorphosed rocks change mineralogy or texture in response to a temperature increase. If the metamorphism is gradual and predictable, we call it progressive metamorphism. During progressive metamorphism, a series of reactions occur as the degree of metamorphism increases. Rock mineralogy changes multiple times before equilibrating at the highest temperature conditions. While this idea makes a convenient conceptual model, it is not correct for all metamorphic rocks. For example, many metamorphic rocks are deep in Earth where pressure and temperature are great. They were never unmetamorphosed rocks at low pressure and temperature. Other rocks go from low temperature to high temperature, perhaps because of rapid intrusion of a pluton, so rapidly that they skip intermediate stages. Still other rocks may only partially equilibrate during metamorphism.
Some metamorphic rocks form by retrograde reactions (metamorphism causing high-temperature rocks to change into low-temperature rocks). This is especially true for mafic rocks that were metamorphosed at high-grade conditions. Upon uplift and cooling, retrograde metamorphism may replace original high-temperature mineral assemblages with low-grade minerals. Figure 8.11 compares paths of prograde and retrograde metamorphism.

One of the most intriguing questions about metamorphic rocks is: Why do we find high-grade metamorphic minerals at Earth’s surface where they are unstable? The Laws of Thermodynamics say that rocks will change mineralogy in response to increasing temperature (prograde metamorphism), so why don’t they undergo opposite (retrograde metamorphism) changes when temperature decreases as the rock reaches Earth’s surface? If rocks always went to equilibrium, we should have no samples of high-grade rocks or minerals to study. Yet, we do. For example, we have samples of diamond-bearing kimberlite, like the specimen seen in this photo (Figure 8.12), that are unstable and should break down at Earth’s surface.
Several considerations help answer these questions:
• Prograde metamorphic events are usually of much longer duration than retrograde events, giving minerals more time to achieve equilibrium.
• Prograde metamorphism liberates fluids not present when retrogression occurs. The fluids act as fluxes to promote prograde metamorphism; their absence may hinder retrogression. And, the absence of fluids means that some low-grade minerals cannot form.
• Prograde reactions are mostly endothermic, which means they consume heat. The heat that causes metamorphism naturally fuels the reactions. In contrast, retrograde reactions are mostly exothermic – they give off heat. There is no outside energy driving the reactions.
• At low temperature, reactions are very sluggish; they may not have time to reach equilibrium.
• More complex, low-grade minerals often have difficulty nucleating and growing.
8.2 Pressure and Temperature
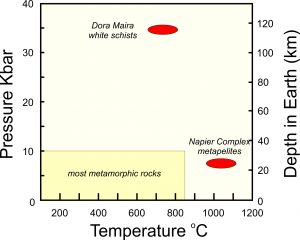
Metamorphism, which may affect any kind of rock, occurs over a wide range of pressure and temperature conditions. This leads to tremendous variation in metamorphic rocks and the minerals they contain. Most of the metamorphic minerals we see form at temperatures of 150 to 850 °C and at pressures of 1 bar to 10 kbar. Exceptions, however, do exist (see Box 8.1, below).
8.2.1 Heat and Temperature
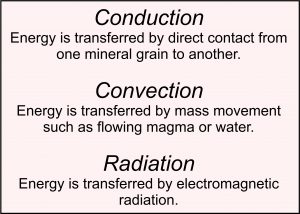
Heat is, perhaps, the most significant cause of metamorphism. Heat is thermal energy that can move (flow) from one place to another or from one substance – such as rock, magma, or water – to another. Thermal energy is high for substances at high temperature and low for substances at low temperature.
Three processes transfer heat: conduction, convection, and radiation; but within Earth, heat transfer by radiation is insignificant. Conductive heat transfer occurs when heat flows naturally from a place of high temperature to one of low temperature with no associated movement of matter. Thus, for example, heat is always flowing from Earth’s hot interior to the cooler surface by conduction. And if a (hot) magma intrudes the (cooler) crust, the magma will cool as heat is conducted grain-by-grain into the surrounding rock, causing the surrounding rock to warm while the magma cools. This warming initially occurs only next to the magma body, but, over time, heat is conducted farther away. Magmatic intrusions can affect a large area.
Convective heat transfer, which is more efficient than conductive heat transfer, is the transfer of heat due to the flow of material, such as the flow of hot water or hot air. Within Earth, convection occurs mostly because of flowing water and flowing magmas. Heat transfer by water can have a significant, although generally quite local, effect. Heat transfer by convecting magmas can be much more significant and can warm huge regions of the crust. And in Earth’s mantle, the slow creep of solid rock due to plate tectonics also moves heat by convection.
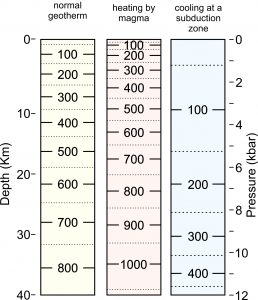
8.17 The temperature increase with depth in Earth in various settings. The numbers in the columns are temperature in ̊C.
Different places on Earth get their heat by different combinations of conduction and convection. Earth’s geothermal gradient, the rate at which temperature increases with depth, averages about 25 to 35 oC/km near the surface in most places. This gradient, also called a geotherm, is mostly due to conductive heat flow. The left column in Figure 8.17 shows temperature-depth relationships for a normal geotherm typical of regions where all heat transfer is by conduction.
In mountain belts and other places where volcanic activity occurs, convective heat flow due to rising magmas contributes much more heat than normal conduction. Consequently, temperature increases faster with depth than is normal (middle column, Figure 8.17). In some places, next to large igneous intrusions, contact metamorphism occurs and extremely high temperatures may persist for short times before the intrusions cool.
In subduction zones (right column, Figure 8.17), generally cooler temperatures are present. Descending slabs of wet cool ocean lithosphere, which have been continuously carried to depth for millions of years, cool the crust and upper mantle below. So, the rate of temperature increase with depth is less than normal.
8.2.2 Pressure and Depth
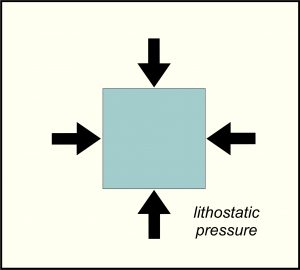
8.18 Lithostatic pressure
After heat, pressure is the most significant cause of metamorphism. If pressure is applied to a rock, the rock may change size or texture, or perhaps develop new minerals to replace old ones. Burial causes rocks to experience lithostatic pressure, also called confining pressure. Lithostatic pressure is the same in all directions (Figure 8.18), and thus can cause an object to become smaller without altering its overall shape. This kind of pressure is equivalent to the pressure that swimmers feel on their ears when they go to the bottom of the deep end of a swimming pool. The pressure on a swimmer’s ears accrues because of the weight of water pushing down from above. Within Earth, the weight of rock, which is commonly three times denser than water, causes lithostatic pressure to build up quickly with depth. As shown in Figure 8.17, pressure of around 12 kbar is reached at 40 kilometers depth, although pressure depends, in part, on the density of overlying rocks. Although typical metamorphic rocks form at pressures of 0 to 10 kbars, we find higher pressure rocks in some places. These high-pressure rocks are rare because to get to very high pressure requires that rocks are buried to great depth – an uncommon occurrence. Subsequently, getting the rocks back to the surface so we can see them is even more problematic.
8.2.3 Directed Stress
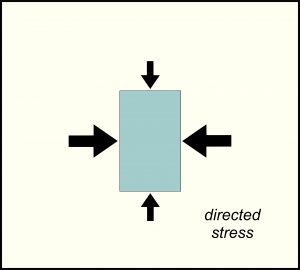
8.19 Directed stress causes deformation
Directed stress, sometimes called differential pressure, is also a force applied to an object, but the force is not the same in all directions. For example, when we squeeze a lemon, we are applying directed stress. When we stretch a rubber band, we are also applying directed stress. The drawing in Figure 8.19 shows greater stress being applied horizontally than vertically, causing compression in one dimension. Within Earth, directed stress is common due to plate tectonic processes that push large pieces of lithosphere together or pull pieces apart. Unlike lithostatic pressure, high levels of directed stresses are not sustained for long because rocks deform to reduce the stress. Directed stress, thus, is commonly associated with rock folding or faulting.
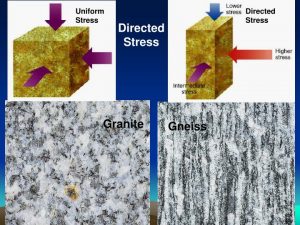
8.20 Gneiss may be created when directed stress is applied to a granite
Directed stress can cause new minerals to form within a rock, but much more commonly it produces deformation, fracturing, or textural changes only. Mineral grains may rotate, align, become distorted, or disintegrate. Figure 8.20 shows how directed stress can change granite (igneous rock) into gneiss (metamorphic rock).
Directed stress may also cause recrystallization as grains dissolve and regrow in other places, or combine to produce larger crystals. Sometimes, directed stress causes shearing and dynamic metamorphism when different parts of a rock slide past each other.

8.21 A mylonite, a highly deformed rock from Otrøy in the Western Gneiss Region, Norway
During shearing, mineral grains can become highly stretched in one direction, and fractures can develop that give a rock a planar texture. This figure (8.21) shows a rock called mylonite, a highly deformed kind of rock created when fine sheared material recrystallizes. The sample is from Norway’s Western Gneiss Region. Directed stress, parallel to the layering in this rock, caused feldspar (white) and biotite (black) grains to become elongated as shearing took place. While this was occurring, metamorphism produced wine-red garnet crystals – a single large one is near the left side of the photo and many small ones are scattered throughout. The 1-euro coin is 2.3 cm across, for scale.
8.2.4 Metamorphic Fluids
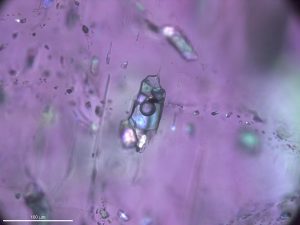
8.22 Fluid inclusions in a crystal of zoisite
Metamorphism often involves fluids, most commonly water-rich but sometimes dominated by carbon dioxide, sulfur, or other components. Many mineral grains contain fluid inclusions that have trapped samples of fluids that once flowed through them. Figure 8.22 shows a fluid inclusion that contains liquid, gas bubbles, and minerals that crystallized from the trapped fluid. The color is an artifact of the way the photo was taken.
Metamorphic fluids may be magmatic (expelled by magmas as they crystallize), meteoric (derived from precipitation that infiltrates the ground), released during subduction of wet lithosphere, or products of reactions that release H2O or CO2 from minerals. Hydrothermal metamorphism occurs when warm fluids significantly alter protolith rocks. This kind of metamorphism can affect large areas and be part of regional metamorphism, or it can be localized and part of contact metamorphism. In either case, the metamorphism involves hot, generally water-rich fluids that flow through cracks and along grain boundaries. The fluids act as catalysts and fluxes that promote reactions and large crystal growth. More important, the fluids may cause metasomatism that changes the composition of the protolith by adding or removing specific elements. The resulting rock may be of a much different composition than its parent. Metasomatism creates many kinds of products. It can, for example, create ore deposits by concentrating minerals (most commonly copper, iron, or lead sulfides) in host rocks where they did not exist previously.
8.3 Metamorphic Textures
8.3.1 Grain size and Porphyroblasts

8.23 A garnet-muscovite schist from Syros, Greece. The garnet porphyroblasts are nearly as large as the 1-euro coin (2.3 cm across).
Textural changes take place as rocks undergo prograde metamorphism, and rocks may develop metamorphic fabrics. A general coarsening of grain size is typical as small mineral grains recrystallize to form larger ones. This is Ostwald ripening in action (refer to the discussion in Section 4.4.2, Chapter 4). While minerals that are already present recrystallize, new metamorphic minerals may grow and modify rock texture. If minerals develop into large crystals that contrast in size with other minerals in a rock, we call the large crystals porphyroblasts. Fine-grained material around the porphyroblasts is the groundmass. The garnets in Figure 8.23 are good examples of porphyroblasts surrounded by groundmass.
8.3.2 Lineations and Foliations
Porphyroblasts are one kind of metamorphic fabric, but there are others. In some deformed rocks, mineral grains assume a distinctive arrangement that gives metamorphic rocks a lineation, long mineral grains all pointing in the same direction, or a foliation, minerals lining up to give a planar fabric. Lineation occurs when amphiboles, kyanite, sillimanite, and other minerals that form long thin crystals, lie parallel in a rock. The photo below in Figure 8.24 shows lineation caused by aligned hornblende (amphibole) crystals.
|
|
|
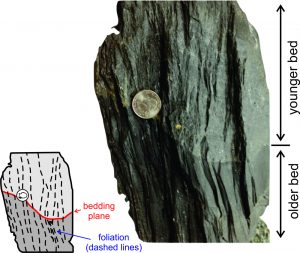
Alignment of clays, micas, graphite, or other platy minerals, the separation of a rock into light and dark layers, or parallel fracturing leads to planar fabrics called foliation. The photo on the right above (Figure 8.25) shows foliation (vertical fracture traces) that cuts across a bedding plane separating older and youger rock layers of different compositions.
8.3.3 Slate

Slates, which form during low-grade metamorphism of shales, comprise primarily microscopic clay grains, perhaps with some minor mica. Metamorphism may obliterate the original bedding as foliation develops perpendicular to the direction of maximum stress. This foliation, slaty cleavage, gives slates a property called fissility – an ability to break into thin sheets of rock with flat smooth surfaces. The photo in Figure 8.25 above shows an example of slaty cleavage. Figure 8.26, seen here, is another example of slate. The minerals in this rock cannot be identified in hand specimen, but in thin section quartz, feldspar, and chlorite can be seen. Slates come in many colors, but various shades of gray are most common. Thin sheets of slate have historically been used for paving or roofing stone.
8.3.4 Phyllite

Figure 8.27 shows a sample of phyllite, a shiny foliated rock created by further metamorphism of slates. The foliation is due to parallel alignment of very small – mostly microscopic – muscovite, chlorite, or other micas, sometimes with graphite. Phyllites, which form at higher metamorphic grades than slates, sparkle because clay minerals have metamorphosed to produce small grains of reflective mica. Thus, foliation of phyllites is different from the foliation in slates that stems from clay mineral alignment, and different from foliation in schists because schists always contain visible mica grains. Like slates, phyllites exhibit fissility.
Phyllites are typically black, gray, or green, and the fine-grained micas and graphite, which are too small to see without a microscope, give phyllites their silky/shiny appearance, or sheen, called a phyllitic luster. It is this luster – which is absent from slate and schist – that really defines a phyllite. Additionally, although not seen in Figure 8.27, layering in some phyllites is deformed, giving the rocks a sort of wavy or crinkly appearance.
8.3.5 Schist

Schists, which form under medium-grade metamorphic conditions, contain medium-to-coarse flakes of aligned mica that we can easily see. This photo (Figure 8.28) shows a typical schist. Schists are higher- grade rocks than phyllites, and most form when phyllites are further metamorphosed. Thus, the precursors of schist are shale, slate, and phyllite. Less commonly, however, schist may form by metamorphism of fine-grained igneous rocks, such as tuff or basalt. Large and aligned flaky minerals, easily seen with the naked eye, define schists. These minerals are most commonly muscovite (such as in this photo) or biotite in parallel or near-parallel orientations that give the rocks schistosity – the ability to be broken easily in one direction but not in other directions.
Most schists are mica schists, but graphite, talc, chlorite, and hornblende schists are common. Quartz and feldspar are present in mica schists, often deformed or elongated parallel to the micas, and many other minerals are possible. If schists contain prominent minerals, we name them accordingly. So the schist in Figure 8.23 is a garnet schist, and the one in Figure 8.28 is a muscovite schist, or simply a mica schist. Photos of staurolite schist and kyanite schist are included later in this chapter (Figures 8.45 and 8.46).
8.3.6 Gneiss
At higher grades, metamorphic rocks may develop compositional layering because different minerals concentrate in layers of contrasting colors. We call such rocks gneisses. The defining characteristics of most gneisses, such as the gneisses seen in Figure 8.29 and Figure 8.30, are that the rocks are medium- to coarse-grained and contain alternating layers of light and dark-colored minerals that give the rock foliation called gneissic banding. The banding in the garnet gneiss (Figure 8.30) is not particularly well-developed but is present.
|
|
Gneisses, the highest temperature-pressure kinds of foliated metamorphic rock, typify many regions that have undergone high-temperature metamorphism. Gneissic banding most commonly forms in response to directed stress. Sometimes, however, layering may form solely due to chemical processes that concentrate different minerals in different layers. The felsic light-colored layers typically contain quartz and feldspars, and the more mafic darker layers typically contain biotite, hornblende, or pyroxene. Accessory minerals such as garnet are common.

Sometimes gneissic banding is deformed, as seen in Figure 8.31. This gneiss, from the Czech Republic, contains pink K-feldspar rich layers alternating with darker layers that contain biotite. Metamorphism produced parallel layers of contrasting mineralogy (and color) and subsequent deformation caused the layers to become deformed. Figure 8.5 shows another example of a deformed gneiss.
Gneisses are often named based on their protoliths, and petrologists use the general terms orthogneiss for gneisses derived from igneous rocks, and paragneiss for gneisses derived from sedimentary rocks. More specific names abound – for example, pelitic gneisses form by metamorphism of originally clay-rich sedimentary rocks, granitic gneisses (such as the one shown in Figure 8.31) form by metamorphism of granites, and mafic gneisses form by metamorphism of mafic igneous rocks. Sometimes key minerals are often included in rock names. For example, a garnet gneiss is a gneiss that contains conspicuous garnet crystals.

Some gneisses do not display well-defined dark- and light-colored banding but still maintain less distinct foliation. For example, the foliation in kyanite gneiss may come from alignment of light-colored kyanite crystals in an otherwise quartz- and muscovite-rich rock. An augen gneiss, such as the gneiss shown in Figure 8.32, contains large feldspar crystals – “eyes” (augen is German for eyes) – stretched in one direction. The gneiss in this photo is oriented so the stretch direction (and, thus, the foliation) is horizontal.
8.3.7 Nonfoliated Metamorphic Rocks

Some metamorphic rocks are fine-grained and lack metamorphic fabrics. For example, hornfels are dark colored fine-grained rocks lacking both lineation and foliation. Many hornfels form at low pressure from contact metamorphism of a mudstone or shale. These rocks may contain no visible layering or fractures and appear as a homogeneous mass. Most hornfels are quite hard and durable because constituent grains are tightly bound together. Figure 8.33 shows an example of biotite hornfels, the most common kind of hornfels. These rocks are dark brown and sometimes have a slight sheen due to microscopic grains of biotite. Other hornfels may have different colors; the color depends on the minerals present. Some hornfels contain grains that become visible after weathering (because different minerals weather in different ways) but, because of the generally uniform rock color, are invisible otherwise.
Greenstones, which are a specific kind of hornfels, form by metamorphism of basalts. Figure 8.34 shows a 9-cm wide sample of greenstone from Ely, Minnesota. The greenish color is due to chlorite or epidote that grew during metamorphism. Figure 8.35 shows an outcrop of greenstone in Italy. The rock originated as an ocean-floor basalt, and contains rounded structures called pillows, indicative of submarine eruption.

Many nonfoliated metamorphic rocks are dominated by a single mineral. In these rocks, individual mineral grains or crystals, which may start small, recrystallize (grow together) during metamorphism to produce larger crystals. Figure 8.36, for example, shows an 8-cm wide rock consisting only of coarse blue calcite. This rock had a limestone protolith. Petrologists use the term marble for all metamorphic carbonate rocks – rocks that form from limestone or dolostone – dominated by calcite or dolomite. (This sometimes leads to confusion because builders and others use the same word to describe any polished slab of rock.)

Quartzite, also a common nonfoliated metamorphic rock, forms by metamorphism of sandstone. Most sandstones comprise mainly quartz and so do quartzites. Figure 8.37 shows a typical example. It consists of small quartz crystals that have grown together so that no grain boundaries are visible without a microscope. The recrystallization produced a typical hard and shiny quartzite (sometimes described as frosty), and during metamorphism any original sedimentary textures were erased. Common quartzites are white or gray, but minor components may add color. The pink color in this sample comes from hematite that may have been part of the cement that held the sandstone together. If the protolith sandstone contained minerals besides quartz, so too will the product quartzite. Thus, feldspar, titanite, rutile, magnetite, or zircon may be present in small amounts. And, if the protolith contained some clay, micas and other aluminous minerals may be present.

This figure (8.38) shows an example of a garnet granulite. Many granulites are foliated, but this one is not. Granulites form at the highest grades of metamorphism and can form from many sorts of protoliths. This rock contains black biotite, light-colored K-feldspar and many conspicuous red garnets. The abundant biotite and garnet tell us that the rock is aluminum-rich, suggesting it has a sedimentary origin. Figure 8.10 shows a different granulite; it contains hornblende and plagioclase besides large garnets. The garnet porphyroblasts in Figure 8.10 are 1-2 cm wide. The garnets in this granulite are only a few millimeters wide at most.
8.4 Metamorphic Reactions
8.4.1 Different Kinds of Reactions
| Examples of Metamorphic Reactions |
| Solid-solid reactions:
xxxandalusite = sillimanite xxxgrossular + quartz = anorthite + 2 wollastonite Dehydration reactions: xxxmuscovite + quartz = K-feldspar + sillimanite + vapor xxxkaolinite + 2 quartz = pyrophyllite + vapor Hydration reaction: xxxenstatite + 2 H2O = 2 brucite + 2 quartz Carbonation reaction: xxxforsterite + 2 CO2 = 2 magnesite + quartz |
As discussed in Chapter 4, under any pressure and temperature, the most stable mineral assemblage is the one with the lowest Gibbs free energy. So, when a rock is heated or squeezed, chemical reactions occur that may consume old minerals and create new ones. These reactions may be of several types. The table seen here gives examples of different types of metamorphic reactions. By convention, the low-temperature mineral or assemblage is to the left of the equal sign; the high-temperature products are to the right.
Solid-solid reactions involve no H2O, CO2, or other vapor phase. The first example of a solid-solid reaction contains only two minerals, both Al2SiO5 polymorphs. This reaction may occur when a metamorphosed shale is heated to high temperature. But most metamorphic reactions involve more than two minerals, and many involve H2O or CO2. The second solid-solid reaction is more typical and involves four minerals.
Dehydration reactions and decarbonation reactions, such as the examples in this table, liberate H2O and CO2, respectively. Hydration reactions and carbonation reactions consume H2O and CO2, respectively.
Metamorphic reactions involve changes in mineralogy or in mineral composition. A mineral assemblage is at chemical equilibrium if no such changes are occurring. If the assemblage has the lowest Gibbs free energy possible for the given conditions, it is at stable equilibrium. In principle, all rocks tend toward stable equilibrium. Whether they reach it depends on many things, including temperature, grain size, and reaction kinetics. If reactions cease before a rock has reached stable equilibrium, the rock is at metastable equilibrium. Many metamorphic rocks contain metastable minerals.
We call a stable mineral assemblage representative of a given set of pressure-temperature conditions a paragenesis. When conditions change, metamorphic reactions may create a new paragenesis as some minerals disappear and others grow. Such reactions may be prograde or retrograde. Most of the reactions in the table above are prograde, but the two examples of carbonation and hydration reactions are retrograde reactions (involving orignial high-temperatue minerals reacting to form low-temperature minerals) that often affect mafic rocks.
Prograde metamorphism involves the breakdown of minerals stable at lower temperature to form minerals stable at higher temperature. Some prograde reactions are solid-solid reactions, but most involve the release of H2O or CO2 that flow along cracks or grain boundaries. As temperature increases, minerals containing H2O or CO2 become increasingly unstable, causing dehydration or decarbonation, and the release of H2O or CO2 as intergranular fluid. If we ignore H2O and CO2, we find that most prograde metamorphism is nearly isochemical, meaning that the rock is the same composition before and after metamorphism. Sometimes, however, flowing fluids and metasomatism can be the dominant forces controlling metamorphism.
Retrograde metamorphism is, in many ways, just the opposite of prograde metamorphism. Typically, H2O- and CO2-free minerals react with fluids to produce hydrous or carbonate minerals. Mg-silicates such as forsterite (Mg2SiO4), and enstatite (Mg2Si2O6), for example, may react to form talc or serpentine (both hydrated Mg-silicates), brucite (Mg hydroxide), or magnesite (Mg carbonate), at low temperature. In contrast with prograde reactions, retrograde reactions are often quite sluggish. They may not go to completion and frequently do not reach stable equilibrium. Sometimes retrogression only affects parts of a rock or parts of some grains in a rock.
8.4.2 Metamorphic Phase Diagrams
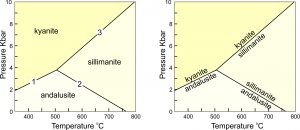
8.39 The stability of different aluminosilicate minerals
The Laws of Thermodynamics allow us to predict which minerals form under particular conditions. We use phase diagrams like the ones seen in Figure 8.39 to show the conditions at which particular minerals or mineral assemblages are stable. The diagram on the left, for example, depicts stability fields for kyanite, sillimanite, and andalusite, the Al2SiO5 polymorphs. The different fields are the ranges of pressure and temperature where each polymorph is stable. The reaction lines separating the fields show the conditions at which chemical reactions occur.
The diagram on the right shows the same information, but the reactions are labeled, not the stability fields. Petrologists use both kinds of diagrams. These diagrams tell us that rocks containing kyanite form at low temperature and high pressure, rocks containing andalusite form at low pressure, and those containing sillimanite form at high temperature. The diagram also allows us to make predictions: for example, if a rock containing andalusite is metamorphosed at high temperature, the andalusite will change into sillimanite.
Phase diagrams for simple chemical systems may only contain a few reactions. The aluminosilicate diagram (above in Figure 8.39) is an example. All the minerals considered have the same composition and are related by three reactions:
kyanite = andalusite (reaction 1)
Al2SiO5 = Al2SiO5
andalusite = sillimanite (reaction 2)
Al2SiO5 = Al2SiO5
kyanite = sillimanite (reaction 3)
Al2SiO5 = Al2SiO5
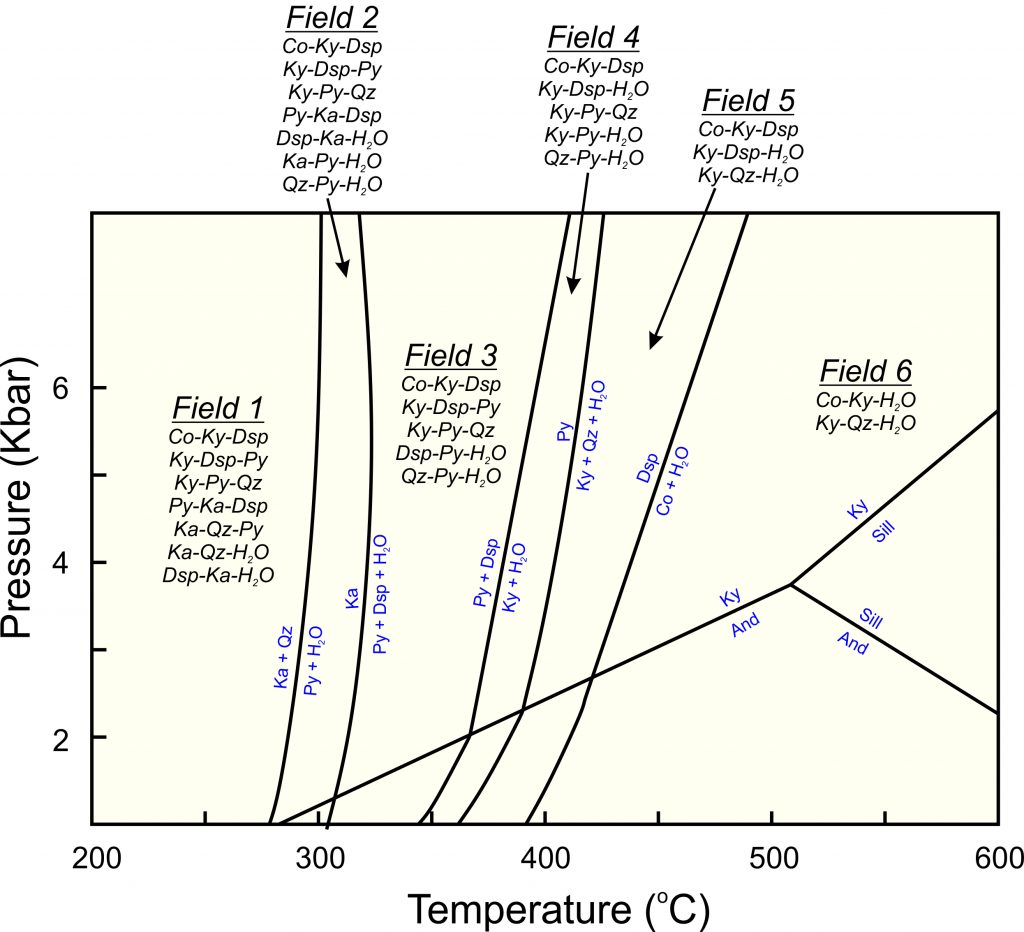
Reactions between kyanite, andalusite, and sillimanite are only three of many that involve minerals of Al2O3, SiO2, and H2O chemical system. Other minerals consist of these same components. The phase diagram seen below in Figure 8.40 is a more complete phase diagram for the system. It includes all stable minerals and reactions. Some of the reactions give off water vapor, labeled as H2O. Eight minerals are involved but most have restricted stability fields.
| Stable Minerals in the System: Al2O3 – SiO2 – H2O |
|
| Mineral | Formula |
| kaolinite (Ka) quartz (Qz) pyrophyllite (Py) diaspore (Dsp) kyanite (Ky) andalusite (And) sillimanite (Sill) corundum (Co) |
Al4(Si4O10)(OH)8 SiO2 Al2Si4O10(OH)2 AlO(OH) Al2SiO5 Al2SiO5 Al2SiO5 Al2O3 |
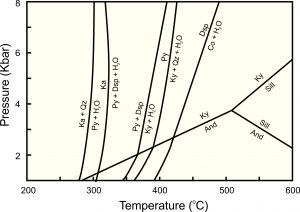
8.40 Minerals and reactions in the system Al2O3 – SiO2 – H2O
According to this phase diagram, at low temperature, kaolinite (a clay mineral) and quartz are stable together, but as temperature increases to almost 300 ̊C, kaolinite and quartz react to produce pyrophyllite and H2O vapor. With a bit more heating, any leftover kaolinite decomposes to pyrophyllite, diaspore, and H2O vapor. Pyrophyllite itself breaks down at higher temperatures – around 400 ̊C, so pyrophyllite is only be stable over a limited range of temperature. At temperatures over about 450 °C, the only stable minerals are corundum, quartz, and the three Al2SiO5 polymorphs (andalusite, kyanite, sillimanite), but corundum and quartz cannot be found together.
Phase diagrams like this permit prediction of the pressures and temperatures at which individual minerals and specific mineral assemblages will form. Conversely, these diagrams allow us to estimate the pressure and temperature of formation for some rocks containing specific minerals. For example, rocks containing kaolinite and quartz are constrained to have formed at temperatures below about 300 °C (because kaolinite is not stable at higher temperature). If andalusite accompanies the kaolinite and quartz, pressure is restricted to less than 1 kbar. The rock must have equilibrated at conditions within the small triagular field near the 300o lable on the bottom axis.
8.5 Metamorphic Rocks of Different Compositions
| Compositional Classes of Metamorphic Rocks | |
| rock class | protolith |
| metapelites | shale and related sediments |
| metasandstones | sandstones |
| metacarbonates | limestone or dolostone |
| metamorphosed iron formation | iron-rich sediments |
| metagranites | granitic rocks |
| metabasalts | basaltic rocks |
Metamorphic rocks may contain all the minerals common in sedimentary and igneous rocks, plus many minerals exclusive to metamorphic rocks. The two most important factors controlling mineralogy are the composition of the rock and the pressure-temperature conditions of metamorphism. For convenience, we divide the most common rock types into general compositional classes. The table seen here lists the most important classes considered by petrologists.
The composition of a metamorphic rock, which is the composition of the protolith, is key because it controls the metamorphic minerals that may be present. Metamorphic minerals in metapelites, metacarbonates, metabasites, metagranites, etc., are all different because of differences in rock chemistry. Metapelites typically contain micas and may also contain staurolite, garnet, and other aluminous minerals. Besides containing calcite or dolomite, metacarbonates may contain Ca-Mg silicates. Metagranites usually contain the minerals that igneous granites contain. And, metabasites commonly contain plagioclase, pyroxenes, and amphiboles. In the discussions below, we look at the minerals common in rocks of different compositions. The focus is mostly on prograde minerals, but rocks of any composition may undergo retrograde metamorphism or alteration that produce a variety of low-temperature minerals.
8.6 Metasedimentary Rocks
8.6.1 Metamorphosed Pelitic Rocks (Metapelites)
Metapelites derive from the metamorphism of shale and other clay-rich sediments. When metamorphosed, dehydration reactions change clay minerals into new minerals containing less H2O. At low grade this leads to the formation of chlorite and muscovite. At higher grade, biotite forms. Foliated textures develop as muscovite and biotite crystallize, so metapelites may be slates, phyllites, schists or gneisses depending on grade. Examples are shown earlier in this chapter in Figures 8.23 and 8.26 through 8.30.
Metapelites are rich in Al, Si, and K and may contain substantial amounts of Fe and Mg, so minerals containing these elements dominate metapelitic rocks. The table below lists the most common and important minerals in these rocks. The minerals in the left column may exist in low-grade rocks, those in the right column are exclusively in high-grade rocks, and the ones in the middle are generally in medium-grade rocks.
| Common Minerals in Metapelites | ||
| low grade | → | high grade |
| quartz SiO2 |
muscovite KAl2(AlSi3O10)(OH)2 |
staurolite (Fe,Mg)2Al9Si4O23(OH) |
| kaolinite Al4(Si4O10)(OH)8 |
kyanite Al2SiO5 |
cordierite (Mg,Fe)Al4Si5O18 |
| pyrophyllite Al2(Si4O10)(OH)2 |
andalusite Al2SiO5 |
K-feldspar KAlSi3O8 |
| chlorite (variable chemistry) |
biotite K(Mg,Fe)3(AlSi3O10)(OH)2 |
sillimanite Al2SiO5 |
| chloritoid (Fe,Mg)2Al4Si2O10(OH)4 |
garnet (almandine) (Ca,Fe,Mg,Mn)3Al2Si3O12 |
orthopyroxene (Mg,Fe)2Si2O6 |
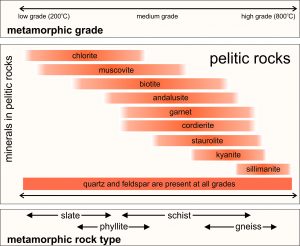
The chart in Figure 8.42 shows typical minerals in metapelites at different grades. Quartz and Na-rich plagioclase are in rocks of all grades. Kaolinite, pyrophyllite and chloritoid may also be present at low grade but are less common and are omitted from this figure. There is a great deal of overlap and many of these minerals exist together. Some persist over a wide range of temperatures. Some are present in low-pressure rocks but not in high-pressure rocks. Either muscovite or biotite are generally present except at the highest grades.
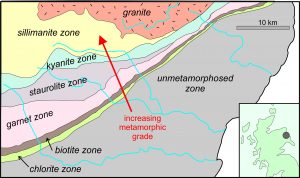
The most classic example of regionally metamorphosed pelites is in the Scottish Highlands where, in the late 19th and early 20th centuries, George Barrow mapped a large region of variable metamorphic grade. The map seen in Figure 8.43 derives from Barrow’s work. In this region, metamorphic grade increases from southeast to northwest. Barrow recognized that the higher-grade metamorphic rocks he was mapping were once unmetamorphosed shales. He mapped different metamorphic zones based on the metamorphic minerals that were present. Each of Barrow’s zones is characterized by a particular index mineral that reflects metamorphic grade. Thus, the zone names in this map.
Rocks similar to the ones described by Barrow, are found worldwide. They are said to be the results of Barrovian metamorphism, a tribute to Barrow. In North America, Barrovian metamorphism is particularly well exposed and studied in the Appalachian Mountains and in the Canadian Shield. Most metapelites in Scotland experienced Barrovian metamorphism, but in an area just north of Aberdeen, metamorphism occurred at slightly lower pressures than classic Barrovian metamorphism. We call this kind of metamorphism Buchan metamorphism, named after the Buchan region where it is found. Besides Scotland, other classic occurrences are in Japan and Spain, but Buchan terranes are found worldwide. Metamorphic rocks in these areas may contain cordierite and andalusite, two low-pressure minerals commonly absent from Barrovian terranes.
The photos below show typical medium-grade metapelites. Figure 8.44 contains garnet porphyroblasts and Figure 8.45 contains staurolite porphyroblasts. The porphyroblasts in both photos are centimeters across. Muscovite surrounds them. Figure 8.46 contains conspicuous blades of blue kyanite surrounded by quartz, some of which is stained reddish by hematite. Figure 8.47 contains centimeter-sized crystals of blue cordierite. Cordierite can be difficult to identify unless it has this diagnostic blue bottle glass appearance. Figures 8.23 and 8.28, earlier in this chapter, also show examples of typical medium-grade metapelites.
|
|
|
|
|
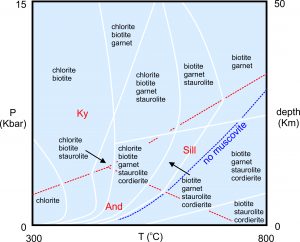
Figure 8.48 shows pressure-temperature stability fields for common pelitic mineral assemblages. Besides the minerals listed, quartz and plagioclase are present under all conditions. The bounding white lines are really diffuse boundaries and the reactions that relate one assemblage to another are complex.
Many metapelitic rocks contain an Al2SiO5 polymorph (andalusite, kyanite, or sillimanite) besides the mineral listed. Red lines and text in this phase diagram show the stability fields for the different polymorphs: kyanite at high pressure, sillimanite at high temperature, and andalusite at low pressure. At medium- or high-grade, Barrovian metamorphism often yields rocks containing kyanite or sillimanite with garnet. At lower pressures, Buchan metamorphism may produce rocks with andalusite, and often cordierite instead of garnet. Muscovite is generally absent at the highest temperatures because it dehydrates to K-feldspar, sillimanite, and water vapor (at temperatures above the blue dashed line). Note that the order of minerals with increasing metamorphic grade in this phase diagram, and in Figure 8.42, matches the order of metamorphic zones mapped by Barrow in Scotland (Figure 8.43).
8.6.2 Metamorphosed Sandstones (Metapsammites)

Compared with metamorphosed pelites, metamorphosed sandstones, also called metasandstones or metapsammites, are often nondescript. Normal sandstones are mostly quartz, perhaps with some feldspar. When metamorphosed, they still contain quartz and feldspar because these minerals are stable at all metamorphic grades. At low grades, metasandstones typically appear massive and homogeneous, containing light-colored quartz and feldspar grains. The rock seen here (Figure 8.49) is an example. Sometimes small micas and other dark minerals may be scattered evenly throughout.
At higher grades metasandstone may recrystallize with quartz grains growing together and becoming coarser. This produces a quartzite, a hard, nonfoliated metamorphic rock. In quartzites, the once separate quartz crystals become massive quartz with no visible grain boundaries. As this happens, original sedimentary textures are obliterated. Pure quartzites are generally white or light colored (like the one in Figure 8.49) but iron staining often adds a red or pinkish coloration. Figure 8.37, earlier in this chapter, shows another example of an unremarkable quartzite.
So, many metasandstones have unexciting mineralogy, but if the original sandstone contained some clay, any of the minerals that can be in metapelites may be present. For example, the greenish quartzite seen below (Figure 8.50) contains disseminated green chlorite. And the kyanite quartzite in Figure 8.51 contains conspicuous blades of blue kyanite. Quartz usually dominates, and the amounts of other minerals depend on how much clay was in the protolith. Foliation, typical of metapelitic rocks, is usually lacking in these rocks because micas are generally absent.
|
|
8.6.3 Metamorphosed Limestones and Dolostones (Marbles)

Geologists generally call metamorphosed carbonate rocks marbles, although this term is used in different ways by building contractors and others. The metamorphism of limestone or dolostone composed only of carbonate minerals produces few mineralogical changes. A general increase in grain size may take place – similar to what happens when sandstone turns into quartzite, but no diagnostic minerals can form because of the limited chemical composition and the high stabilities of both calcite and dolomite. The photo in Figure 8.52 shows a marble that contains only course crystals of white calcite. Figure 8.36, earlier in this chapter, showed a marble consisting only of blue calcite.
However, most limestones contain some quartz and other minerals besides carbonates. In these rocks, a series of interesting Ca-silicates, Ca-Mg-silicates, and Ca-Al-silicates form as metamorphism progresses. The table below lists the most important of these minerals, roughly in order of their appearance in response to increasing metamorphic grade.
| Minerals Common in Metacarbonates | ||
| low grade | → | high grade |
| calcite CaCO3 |
talc Mg3Si4O10(OH)2 |
grossular (garnet) Ca3Al2Si3O12 |
| dolomite CaMg(CO3)2 |
tremolite Ca2Mg5Si8O22(OH)2 |
periclase MgO |
| quartz SiO2 |
forsterite (Mg,Fe)2SiO4 |
wollastonite CaSiO3 |
| phlogopite (Mg-rich biotite) K(Mg,Fe)3(AlSi3O10)(OH)2 |
diopside CaMgSi2O6 |
monticellite CaMgSiO4 |
If quartz is present, the metamorphic reactions in marbles are often decarbonation reactions that involve the breakdown of carbonates to release CO2. If a pluton intrudes a limestone or dolostone, contact metamorphism may cause CO2 to flow out of the carbonate and combine with H2O that comes from the pluton. The CO2-H2O fluid can have profound effects on the carbonate nearby, and fluid composition controls the formation of many minerals. Fluids may also cause significant metasomatism and a significant change in rock chemistry.
|
|
|
|
|
Phlogopite is typically one of the first minerals to form during carbonate metamorphism. The first photo in the block above (Figure 8.53) shows large, somewhat hexagonal, flakes of phlogopite with calcite behind. The second photo (Figure 8.54) shows gray blades of tremolite in a marble that also contains small (hard to see) specs of graphite. The bottom left photo (Figure 8.55) show a marble that contains green forsterite (olivine). The last photo (Figure 8.56) shows a marble that contains green diopside. These four photos are in order of increasing metamorphic grade. The diopside marble is the highest grade of the four.
8.6.4 Metamorphosed Iron Formations

Ironstone is a general name we give to sedimentary rocks that contain more than 15% iron. These rocks may contain iron hydroxides (limonite), oxides (magnetite and hematite), carbonates (siderite), or silicates (chamosite, Fe-rich chlorite). They generally have a uniform, nonfoliated texture.
Iron formations are similar to ironstones but are mainly Precambrian (ironstones are Phanerozoic). Iron formations generally contain abundant chert and are often well banded with bands ranging from centimeters to meters thick. The bands consist of alternating iron- and chert-rich layers. Figure 8.57 photo shows an example of iron formation from western Australia.
When ironstones and iron formations are metamorphosed, they quickly lose any original hydrous minerals. But any of the other original minerals may persist. At the lowest grades of metamorphism, magnetite and hematite most commonly dominate. If the original rock was rich in carbonate, siderite (Fe-carbonate) will be present. And sometimes pyrite is present as well. At higher grades, greenalite, minnesotaite, and glauconite (all iron silicates) may form. At still higher grades, metamorphism may produce actinolite, grunerite, hedenbergite, or fayalite. The table below summarizes these relationships.
| Common Minerals in Metamorphosed Iron Formations | ||
| low grade | → | high grade |
| quartz SiO2 |
pyrite FeS2 |
actinolite Ca2(Fe,Mg)5Si8O22(OH)2 |
| hematite Fe2O3 |
greenalite Fe2-3Si2O5OH4 |
grunerite Fe7Si8O22(OH)2 |
| magnetite Fe3O4 |
minnesotaite Fe3Si4O10(OH)2 |
hedenbergite CaFeSi2O6 |
| siderite FeCO3 |
glauconite (K,Na)(Fe,Al,Mg)2(Si,Al)4O10(OH)2 |
fayalite Fe2SiO4 |
The photos below show minerals common in metamorphosed iron formations. The hematite shown in Figure 8.58 is specular hematite (more common hematite has a red earthy color). Actinolite, seen in Figure 8.59, is a calcium-iron amphibole. Grunerite (Figure 8.60) is an iron amphibole. Greenalite (Figure 8.61) is an iron-rich variety of serpentine. Siderite (the brown mineral in Figure 8.62) is an iron carbonate, and pyrite (Figure 8.63) is iron sulfide. The pyrite in Figure 8.63 is somewhat tarnished.
8.7 Metaigneous Rocks and Minerals
8.7.1 Metamorphosed Granitic Rocks
The quartz, K-feldspar, and plagioclase that make up most granites and intermediate igneous rocks are stable at all grades of metamorphism. So, metamorphism of granites may not lead to significant mineralogical changes. However, many granites contain mafic minerals, most commonly biotite and hornblende. These minerals may dehydrate to produce new metamorphic minerals at medium and high grade. The table below lists the most common minerals in metamorphosed granites (also called metagranites). At the highest grade, metagranites become granulites, defined by the presence of orthopyroxene formed by dehydration of mafic minerals. Figure 8.31, earlier in this chapter, showed an example of a granitic granulite. Accessory minerals found in unmetamorphosed granites may also be present after metamorphism. At high grade, granitic rocks sometimes develop gneissic banding, even if mineralogy has not significantly changed.
| Common Minerals in Metamorphosed Granitic Rocks | ||
| unmetamorphosed | low grade | high grade |
| Quartz SiO2 |
biotite K(Fe,Mg)3(AlSi3O10)(OH)2 |
Fe-rich garnet (almandine) Fe3Al2Si3O12 |
| K-feldspar KAlSi3O8 |
hornblende (complex amphibole) |
orthopyroxene (Fe,Mg)SiO3 |

The photo above (Figure 8.64) shows a metagranite from the Western Gneiss Region of Norway. During metamorphism, K-feldspar recrystallized to form very large pink crystals. Gray glassy quartz, white plagioclase, and black biotite are also present. This rock shows a significant amount of deformation, recorded by the deformed sheets of biotite. Note the presence of gneissic banding, most notably to the right of the marker pen.
8.7.2 Metamorphosed Mafic Rocks (Metabasites)
| Common Minerals in Metabasites | |||||
| minerals | rock names | ||||
| low grade
high grade |
zeolites prehnite pumpellyite Ca-rich plagioclase epidote chlorite actinolite hornblende garnet (almandine-pyrope)(Fe,Mg)3Al2Si3O12 biotite augite (pyroxene) orthopyroxene (enstatite) |
igneous
greenstone
amphibolite
mafic gneiss
mafic granulite |
|||
Metamorphosed basalts and other rocks of similar composition are commonly called metabasites. This is because, geologists once called basalts basic rocks. Compared with metasandstones and metapelites, metabasites are relatively poor in Al and Si and rich in Ca, Mg, and Fe. Many different minerals may form, and metamorphic reactions are complex. Plagioclase and augite are stable at all grades but other minerals are not. The most important metamorphic minerals are Ca and Mg silicates. Metabasites are generally more massive and less foliated than pelitic rocks, but at higher grades they do form schist and gneiss.
The table seen here lists the most common minerals in metabasites. Low-grade minerals are at the top of the table, and grade increases downward. Metamorphism often begins with the formation of zeolites, or of prehnite. These minerals may crystallize in vugs or cracks. They are secondary minerals in many igneous rocks, and form by hydration of feldspars when water flows through the protolith. Some petrologists do not consider these minerals to be metamorphic minerals, while others do.
The formation of greenstones is said by many to be the beginning of metamorphism. Greenstones are fine-grained, very low-grade metabasites that have a conspicuous light- to dark-gray or green color. The characteristic green color comes from fine-grained chlorite and epidote in the rocks. Greenstones may also contain Na-rich plagioclase (albite), quartz, carbonates, and zeolites. The photo below in Figure 8.65 shows a typical greenstone outcrop in northern Minnesota. Figures 8.34 and 8.35, earlier in this chapter, showed other examples.
At slightly higher grades, metabasites become greenschists, obtaining schistosity from parallel arrangements of the green amphibole actinolite and chlorite. Figure 8.66, below, shows a greenschist from the Homestake Gold Mine in Lead, South Dakota. Although hard to see, the specimen contains native gold near the bottom of the sample. If you enlarge the photo you can see the gold.
At still higher grade, chlorite, epidote, and actinolite break down by dehydration reactions, producing a specific kind of rock called an amphibolite. The photo in Figure 8.67 is an example. Amphibolites contain large grains of black hornblende and whitish plagioclase in subequal proportions. Garnet, biotite, and light-colored amphiboles such as anthophyllite or cummingtonite may also be present.
With even more metamorphism, mafic rocks may become mafic gneisses. At the highest grades, all amphiboles become unstable and dehydrate to produce pyroxenes. Assemblages including garnet and clinopyroxene, or orthopyroxene, are diagnostic of mafic granulites. Figure 8.10 earlier in this chapter, showed an example of a mafic granulite. Minor minerals at all grades include many that are present in mafic igneous rocks.
8.7.2.1 Metamorphic Facies
Pentti Eskola, a geology professor at the University of Helsinki, introduced the idea of metamorphic facies in 1920. He observed that the equilibrium mineral assemblage and texture of metabasites vary with pressure and temperature. Thus, rock mineralogy and texture record the conditions of metamorphism. Eskola defined facies as general ranges of pressure and temperature characterized by a distinct kind of metabasite.
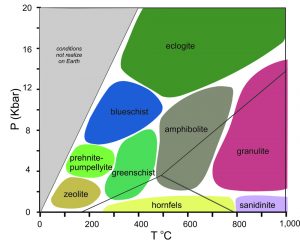
Facies diagrams, such as the one in Figure 8.68, are similar to phase diagrams because they divide P-T space into small areas associated with specific minerals or mineral assemblages. The main differences between facies diagrams and phase diagrams are that facies diagrams involve many chemical components, the locations of different facies in P-T space are not precise, and we often do not know the exact reactions that relate one facies to another. Eskola originally identified eight facies. Other petrologists have divided some to more precisely represent pressure and temperature ranges. Each facies name comes from its most characteristic metabasite minerals or rock types. The table below summarizes key mineral assemblages for each facies.
| Key Mineral Assemblages in Mafic Rocks of Different Metamorphic Facies | ||
| kind of Metamorphism | metamorphic Facies | diagnostic Minerals |
| contact metamorphism | pyroxene hornfels | orthopyroxene + clinopyroxene + plagioclase |
| sanidinite | sanidine or tridymite or pigeonite or glass | |
| low-pressure metamorphism | zeolite | zeolites + quartz |
| prehnite-pumpellyite | prehnite or pumpellyite + quartz | |
| greenschist | chlorite, epidote, albite, quartz | |
| amphibolite | hornblende + plagioclase | |
| granulite | orthopyroxene or garnet + clinopyroxene + quartz | |
| high-pressure metamorphism | blueschist | glaucophane |
| eclogite | omphacite + garnet ± quartz | |
Zeolite minerals and clays characterize the zeolite facies. This facies represents the lowest grade of metamorphism; it is often hard to distinguish zeolite-facies metamorphism from diagenesis. As temperature rises, the zeolite facies gives way to the prehnite-pumpellyite facies, the greenschist facies, the amphibolite facies, and the granulite facies. Contact metamorphism produces two low-pressure, high-temperature facies, the pyroxene-hornfels facies and the sanidinite facies. The blueschist facies and the eclogite facies occur at high pressure.
Eskola based his facies names on minerals and textures of mafic rocks. The names of the facies are names of different kinds of metamorphosed mafic rocks. But petrologists use the same names when talking about rocks of other compositions. This leads to some confusion. The table above lists key mineral assemblages in mafic rocks, but the assemblages will never be present in rocks of other compositions. For example, pelitic or calcareous rocks do not form greenschists (green mafic schists) or amphibolites (mafic rocks dominated by amphibole and plagioclase) even when metamorphosed at conditions within the greenschist or amphibolite facies. In addition, for some rock compositions, several different mineral assemblages may be stable within a single facies. Further confusion arises because petrologists use some facies names in a more restricted sense, referring to particular rock types with important tectonic significance. Despite these problems, the facies concept provides a convenient way to discuss general ranges of pressure and temperature, and it receives wide use.
8.7.2.2 Facies Series
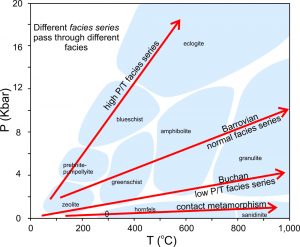
As a model for progressive metamorphism, petrologists consider different metamorphic facies series, the sequences of metamorphic rocks that would form in different metamorphic environments. The PT diagram in Figure 8.69 shows the most important of these series.
Rocks undergoing contact metamorphism experience only low pressure. They pass through the zeolite, prehnite-pumpellyite, low-pressure greenschist, pyroxene hornfels and sanidinite facies with increasing temperature. Such rocks are common anywhere magma has intruded shallow crustal rocks.
Rocks subjected to regional metamorphism during mountain building experience a significant increase in both pressure and temperature. They progress through the zeolite, prehnite-pumpellyite, greenschist, amphibolite, and granulite facies. Sometimes they follow a Buchan facies series (lower pressure) and sometimes they follow a Barrovian facies series (higher pressure).
Subduction carries relatively cool rocks to depth and high pressures. So, some rocks related to subduction zones follow the high P/T facies series, experiencing conditions in the zeolite, prehnite – pumpellyite, blueschist, and possibly eclogite facies. We find these rocks, typically, as blocks in fault contact with greenschist facies rocks. Petrologists have described blueschists from many places, but the two classic examples of the blueschist facies series are rocks of the Sanbagawa metamorphic belt of Japan and of the Franciscan Complex of California.
8.7.3 Metamorphosed Ultramafic Rocks
| Common Minerals in Metamorphosed Ultramafic Rocks | |||
| minerals | |||
|
low grade
high grade |
talc Mg3Si4O10(OH)2 c brucite Mg(OH)2 c magnesite MgCO3 c serpentine Mg6Si4O10(OH)8 c olivine (Mg,Fe)2SiO4 c anthophyllite (Mg,Fe)7Si8O22(OH)2 c garnet (pyrope-almandine) (Mg,Fe)3Al2Si3O12 c clinopyroxene (diopside) CaMgSi2O6 c orthopyroxene (enstatite) Mg2Si2O6 |
||
Ultramafic rocks come from Earth’s mantle. Sometimes, during tectonism, they make it to the surface so we can study them. Most of the examples of metamorphosed ultramafic rocks that we see are in ophiolites, slivers of Earth’s oceanic crust and mantle uplifted and accreted onto continents. The outcrop photo below (Figure 8.70) shows a rock of the Lizard Complex, one of the best-known ophiolites.

Mantle rocks are high-temperature, high-pressure rocks that typically contain olivine, clinopyroxene, and orthopyroxene when unweathered. When weathered or metamorphosed at low temperature, the original minerals often react to create low-temperature minerals. Hydration and carbonation reactions occur and produce hydrous and carbonate minerals. Magnesium oxides and hydroxides may also form. Thus, unless metamorphic temperatures are very high, metamorphism of ultramafic rocks produces low-temperature minerals from high-temperature minerals, essentially retrograde metamorphism.
The table above lists the most common minerals in metamorphosed ultramafic rocks. Low-grade minerals are at the top of the table and high-grade minerals at the bottom. Ultramafic rocks are very Mg-rich, and often contain much olivine. Because of their chemistry, Mg-silicates such as talc, serpentine, anthophyllite, diopside, and enstatite are also common in these rocks.

Low-grade metamorphism or alteration of olivine-bearing rocks often produces a brownish, highly weathered, appearance, such as seen in this outcrop photo (Figure 8.71). Typically, these rocks contain serpentine that developed by hydration of olivine. We call rocks rich in serpentine serpentinites. Figure 8.9, near the beginning of this chapter showed another example. Serpentine has three polymorphs: antigorite, lizardite, and chrysotile. The photos below show examples of each.
|
|
|
Antigorite, the most common serpentine mineral that forms during metamorphism of ultramafic rocks, is stable over a wide range of metamorphic conditions. The weathered outcrop in Figure 8.72 is a typical occurrence. The green color and the texture are diagnostic. Lizardite (Figure 8.73), named for occurrences in the Lizard Complex, Cornwall, and chrysotile (Figure 8.74) are less common and form exclusively at low pressures or at Earth’s surface. The green lizardite in the middle photo above contains pinkish inclusions of stichtite, a rare magnesium chrome carbonate. Chrysotile is one of the few recognized asbestos minerals; fine fibers are easily seen in Figure 8.74. The other asbestos minerals are amphiboles.
While serpentine commonly dominates very low-grade ultramafic rocks, several other minerals may also be present. The photos below show talc (hydrated Mg-silicate), brucite (Mg-hydroxide), and magnesite (Mg-carbonate). These minerals, common in metamorphosed ultramafic rocks, can also form in metacarbonates.
|
|
At higher metamorphic grades, ultramafic rocks may contain olivine, anthophyllite, enstatite, periclase, or spinel. And, at the highest grades, garnet and pyroxene become stable. Minerals in high-grade ultramafic rocks are the same as the minerals in rocks of the mantle (where pressure and temperature are great). In effect, mantle rocks originated as high-grade metamorphic rocks. We saw photos of several examples in Chapter 6 (Figures 6.118, 6.119, and 6.120).
8.8 High-Pressure Rocks and Minerals
| Common Minerals in Metamorphosed High-Pressure Rocks |
|||
| minerals | |||
| low grade
high grade |
glaucophane lawsonite epidote jadeite aragonite kyanite garnet (pyrope-almandine) omphacite |
||
Because of their tectonic significance, petrologists group high-pressure metamorphic rocks into a class unrelated to rock composition. These rocks include mainly blueschists and eclogites, both quite rare. They come from deep in Earth, and special conditions are required to create them and bring them to Earth’s surface. Blueschist is a name given to one type of rock that forms at conditions within the blueschist facies, a facies characterized by high pressure and relatively low temperature. Blueschist chemistry is variable. Compositions range from pelitic to mafic. No matter their compositions, they contain conspicuous mineralogy.
A blue amphibole, called glaucophane, is responsible for the name of the facies. The blueschist seen below in Figure 8.78 is mostly glaucophane. Other common blueschist minerals include a colorless to green Na-pyroxene called jadeite, green or white lawsonite, and pale aragonite (the high-pressure polymorph of calcite). Epidote, garnet, zoisite, quartz, and other accessory minerals may also be present. Because they form at low temperature, blueschists are often fine grained, poorly crystallized, and difficult to study.
Eclogites, such as the one seen below in Figure 8.79, are mafic rocks metamorphosed at high pressure and moderate-to-high temperature. They contain the essential minerals pyrope (Mg-rich garnet) and the green Na-rich clinopyroxene called omphacite. Orthopyroxene may also be present in significant quantities. Accessory minerals include kyanite, quartz, spinel, titanite, and many others. Eclogites originate in the deep crust or in the mantle. Many mantle xenoliths, carried up as nodules within magma, are eclogites. Eclogites are also found as layers or bands associated with some peridotites. Quite commonly, eclogites undergo retrograde metamorphism and so become partially changed into blueschists.
|
|
The photos below show some additional examples of high-pressure minerals. The light-colored crystals in Figure 8.80 are lawsonite. Lawsonite has about the same composition as anorthite. But plagioclase (including anorthite and albite components) becomes unstable at high pressure, so the anorthite part hydrates and we get lawsonite instead. The green jadeite, in Figure 8.81, is an Na-rich pyroxene that is only stable at high pressure. It forms because the albite component in plagioclase changes by solid-solid reaction into Na-pyroxene. Glaucophane, the inky blue mineral in the lower left photo (Figure 8.82) is an Na-rich amphibole. Like omphacite, it incorporates its sodium component from albite. In this specimen, a silvery and greenish chrome mica, fuchsite, accompanies the glaucophane. The green omphacite in the lower right photo (Figure 8.83) is a pyroxene that includes a few high-pressure components. And the pyrope (red garnet) in the same sample formed by solid-solid reactions involving pyroxenes.
white line
white line
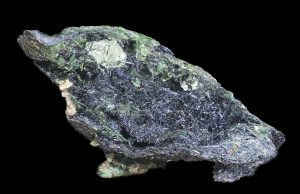
●Figure CreditsUncredited graphics/photos came from the authors and other primary contributors to this book. 8.1 Zoisite, corundum, and hornblende, James St. John, Wikimedia Commons |