4 Crystals and Crystallization
KEY CONCEPTS
- With just a few exceptions, all minerals are crystalline.
- Crystalline substances have an orderly and repetitive atomic arrangement.
- Crystals grow from small seeds and sometimes become very large.
- Igneous minerals precipitate from a magma; most of them are silicates.
- Aqueous minerals precipitate from water; they include compounds of high solubility.
- Hydrothermal minerals precipitate from warm flowing waters.
- Metamorphic minerals form by solid-state reactions during metamorphism.
- Some minerals form during weathering or diagenesis.
- Minerals may not form or be stable under all conditions.
- Minerals may have defects involving misplaced or missing atoms.
- Minerals may be heterogeneous.
- Mineral crystals may be twinned, containing domains with slightly different atomic orientations.
4.1 Crystalline and Noncrystalline Solids
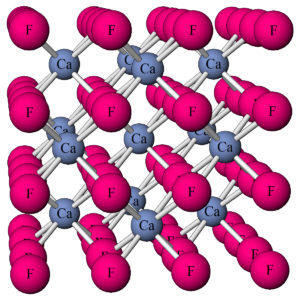
By definition (with just a few special exceptions) minerals must be crystalline. This means that they are solids with an orderly repetitive atomic arrangement. For example, this ball and stick model (Figure 4.2) shows the atomic arrangement in fluorite (CaF2).
Fluorite is one of a small number of common minerals that are isotropic. Isotropic minerals have very symmetrical atomic arrangements and atoms are arranged in an overall cubic pattern. The same atoms are encountered in any direction through the mineral and, consequently, mineral properties are the same all directions. The photo in Figure 4.3 shows light purple cubic fluorite crystals. They are interpenetrating deformed cubes. Many spectacular specimens of cubic fluorite crystals like this one are found in museums.

Other isotropic minerals, besides fluorite, include diamond (C), almandine (Fe3Al2Si3O12), gold (Au), pyrite (FeS), silver (Ag), spinel (MgAl2O4), and sodalite (Na3Al3Si3O12•NaCl). All these minerals, have a cubic arrangement of atoms, although their crystals may not be cubes. Still, their crystals, when euhedral, are equant and generally have many identical faces.
Most minerals, however, are anisotropic. This means that they have different properties in different directions. Consequently, their crystals are not as symmetrical as fluorite’s. Yet, crystals may possess other symmetry, even if they do not resemble a cube.

So, well-formed mineral crystals often exhibit symmetry. But, it may not be cubic symmetry like fluorite – other shapes are possible. For example, the blue-gray barite (BaSO4) crystals seen in this photo are tabular and have a long, an intermediate, and a short dimension. The shape, termed orthorhombic, is similar to the shape of a shoebox.
Barite is an anisotropic mineral. The crystals have lots of symmetry, but it is different from the symmetry of fluorite. Still, when compared with many minerals, barite crystals are quite symmetrical. Olivine, some pyroxenes, and topaz all sometimes have crystal shapes similar to barite’s.

Some mineral-like substances are noncrystalline, also described as amorphous, which means they have a random atomic structure. Natural volcanic glass, obsidian, is an example. This photo (Figure 4.5) shows a specimen of snowflake obsidian. It contains black volcanic glass (obsidian) with white patches. Obsidian is noncrystalline, but over time it will sometimes begin to crystallize, creating the white patches seen here. The patches consist of cristobalite, a variety of SiO2 that is a polymorph of quartz.
Amorphous solids are generally isotropic, so they have the same properties in all directions. This gives the materials properties that are sometimes very useful. For example, window glass is made by melting mixtures containing mostly quartz sand, and allowing the melt to solidify quickly so atoms cannot arrange themselves in a crystalline structure. The process produces a noncrystalline isotropic glass, so light can pass through it equally well in all directions. This is one reason why manufactured glass makes a better window than transparent minerals.
4.2 Forming Crystals
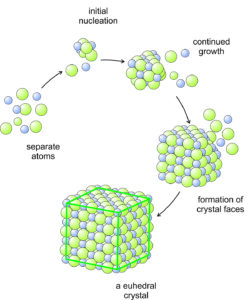
The formation of crystals involves the bringing together and ordering of constituent atoms. Crystals grow from a small single molecule to their final visible form. This can happen in many different ways and settings, but the principle mechanisms are three:
• crystals growing as magma cools
• crystals precipitating from water
• crystals forming by chemical reactions
The drawing shows separate atoms coming together to produce a cubic crystal. In this example, the crystal is euhedral with six identical faces. But, even if it did not develop crystal faces, the atoms could still be in a cubic pattern, and the product material would still be crystalline.
4.2.1 Igneous Minerals
In magma (molten rock), kinetic energy means that atoms are always in motion. The photo in Figure 4.8 shows lava (magma that has reached Earth’s surface) in Hawaii. When magma is at high temperature, it is completely liquid because high kinetic energy ensures that no solid is stable. Some atoms collide and may form bonds temporarily before breaking apart again. A balance exists between the formation of bonds and the rate at which they break apart. If bonds break as fast as they form there will be no net crystallization.
As magma cools, kinetic energy decreases when atoms slow down. Eventually, if magma cools sufficiently, atoms will slow down enough so that some bonds will begin to persist. This is the beginning of the formation of crystals from a melt, and the beginning of the formation of igneous minerals. Initial crystallization creates small nuclei, many of which continue as the centers of crystals during continued growth. Because of high temperatures and the molten state of magma, atoms are quite mobile and easily move toward the nuclei and to surfaces of growing crystals. So crystals may become large, like the dark colored pyroxene and light grey plagioclase in the gabbro in Figure 4.9.
The table below lists some common minerals in igneous rocks. Almost all are silicates because the magmas that produce igneous rocks are dominated by oxygen and silicon. Other abundant elements include aluminum, iron, magnesium, calcium, sodium, and potassium. These elements make up most igneous minerals.
| Common Minerals in Igneous Rocks | ||||
| class or group | minerals or series | chemical formula | ||
| olivine | olivine | (Mg,Fe)2SiO4 | ||
| pyroxene | diopside augite orthopyroxene |
CaMgSi2O6 (Ca,Mg,Fe,Na)(Mg,Fe,Al)(Si,Al)2O6 (Mg,Fe)2SiO6 |
||
| amphibole | hornblende | (K,Na)0-1(Ca,Na,Fe,Mg)2(Mg,Fe,Al)5(Si,Al)8O22(OH)2 | ||
| mica | biotite muscovite |
K(Mg,Fe)3(AlSi3O10)(OH)2 KAl2(AlSi3O10)(OH)2 |
||
| feldspar | orthoclase microcline sanidine plagioclase |
KAlSi3O8 KAlSi3O8 KAlSi3O8 (Ca,Na)(Si,Al)4O8 |
||
| feldspathoid | leucite nepheline |
KAlSi2O6 (Na,K)AlSiO4 |
||
| silica | quartz | SiO2 | ||
| oxide | magnetite ilmenite |
Fe3O4 FeTiO3 |
||
| sulfide | pyrite pyrrhotite |
FeS2 Fe1-xS |
||
| other | titanite zircon apatite |
CaTiSiO5 ZrSiO4 Ca5(PO4)3(OH,F,Cl) |
||
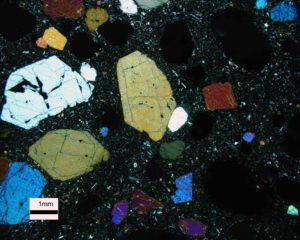
If the conditions are right, igneous mineral crystals may develop prominent crystal faces. The photo here in Figure 4.10 is a microscope view of Hawaiian basalt. It was obtained using a petrographic microscope. The largest gray, yellow, and blue minerals are pyroxene, but their colors, called interference colors, are not the true colors of the mineral. Somewhat smaller minerals with brighter colors are olivine. The pyroxene shows cleavages and the olivine does not. Because they had room to crystallize and grow from a magma, some of the olivine and pyroxene crystals have (imperfectly formed) crystal faces. The black material with fine white flecks in the background is basaltic glass containing fine crystals of plagioclase.
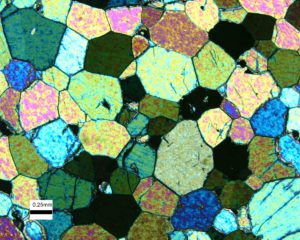
If crystals do not have room to grow individually, and instead crystallize simultaneously, the result may be a rock with crystals forming a mosaic pattern, like the rock seen in Figure 4.11. This view shows grains of olivine in a dunite (a rock composed nearly entirely of olivine). The colors, like the colors in the previous figure, are not true mineral colors but are artifacts of the way this rock was viewed. Olivine, pyroxenes, feldspars, and many other minerals commonly develop this kind of texture.
Igneous processes are quite variable. Some magmas cool slowly underground, so mineral crystals grow to be large. Other magmas extrude as lavas and cool quickly to form basalt or another extrusive rock. Mineral crystals in extrusive rocks may be so small that they cannot be seen with the naked eye or even with a microscope. Silicate minerals dominate igneous rocks but magma compositions vary somewhat. So igneous rocks have variable compositions and consequently variable mineralogies.
4.2.1.1 Pegmatites

Pegmatites are exceptionally coarse grained igneous rocks. In some pegmatites, crystals may be huge. The photo seen here shows white quartz, salmon-colored feldspar and black riebeckite in a pegmatite. The hand lens is about 2 cm across. Pegmatites form during the final stages of magma crystallization. Most have overall composition similar to that of granite.
The largest crystals in the world have been found in pegmatites. A single crystal of mica (phlogopite) from Ontario, Canada, is 4.2 m (14 ft) wide and 10 m (33 ft) long. A quartz crystal from a Russian pegmatite weighs more than 907 kg (2,000 lbs.). The largest quartz crystal on record, however, was from a pegmatite in Brazil and weighed more than five tons.

Some pegmatites are enriched in elements that are normally minor components of magmas. The photo seen here is a crystal of watermelon tourmaline from a pegmatite. Tourmaline is really the only common mineral that contains boron.
Other elements that concentrate in pegmatites include cesium, beryllium, zirconium, niobium, uranium, thorium, tantalum, tin, rare earth elements, chlorine, fluorine, lithium, and phosphorus. Consequently, pegmatites are sometimes mined for these elements. Pegmatites also yield gemstones, including varieties of tourmaline like the one shown, bright green feldspar called amazonite, several varieties of beryl (emerald, aquamarine, and heliodor), and others.
4.2.2 Aqueous Minerals
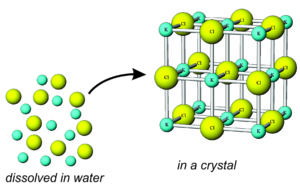
A similar process – similar to crystallization from magma – occurs when minerals precipitate from water to form aqueous minerals. Aqueous means involving water. Because water may contain ions of many different sorts, a number of different aqueous minerals are possible.
Precipitation depends on a number of factors. As long as kinetic energy is high, or water is not saturated, no crystals will form. Ions will bond temporarily, only to break apart and return to solution. This can change, however, if conditions change.
Most substances are more soluble in water at high temperature than at low temperature. So, a decrease in temperature may lead to oversaturation, nucleation, and precipitation of minerals. During this process, unbonded dissolved ions become organized in a crystal structure. For example, K+ and Cl– may combine to form the mineral sylvite (KCl). And, similarly, calcium carbonate precipitates to form calcite (CaCO3) if concentrations of Ca2+ and CO32- in water are high enough.
Crystal precipitation from water may also occur because of chemical change. Suppose, for example, that seawater evaporates. The concentration of dissolved material in the remaining water will increase, leading to oversaturation and, eventually, precipitation of crystals. Besides changes in temperature and composition, changes in pressure, pH, or other things may also lead to the formation of aqueous crystals. The photos below show two examples: halite and gypsum deposits in Utah.
Inland lakes or seas commonly precipitate calcite, halite, gypsum, and other minerals. In some places evaporating waters have deposited salt beds thicker than 300 m. Figure 4.15 shows salt that was deposited by Utah’s Great Salt Lake. Similar deposits are found along the shores of The Dead Sea (between Jordan and Israel) and other seas and lakes in arid regions. On a much smaller scale, minerals precipitating from slowly moving groundwater can fill holes, fractures, and cracks in rocks. Figure 4.16 shows veins of gypsum in Utah’s Moenkopi Formation. Gypsum is commonly associated with red sandstones and mudstones like the rock seen in this photo.
The table below lists some typical aqueous minerals. The most common are minerals with high solubility in water, such as calcite, halite, and other salts.
| Common Aqueous Minerals | ||
| mineral class or group | important minerals or mineral series |
chemical formula |
| silica | quartz | SiO2 |
| carbonate | calcite dolomite magnesite |
CaCO3 CaMg(CO3)2 MgCO3 |
| halide | halite sylvite |
NaCl KCl |
| sulfide | gypsum anhydrite |
CaSO4•2H2O CaSO4 |
| native element | sulfur | S |

Other minerals, having lower solubility but composed of elements in great abundance, slowly form from aqueous solutions. Quartz is an example. At low temperature, quartz may precipitate in geodes such as the one seen here. This geode contains amethyst, a purple variety of quartz, and also more common clear white quartz. Note the concentric layers in this specimen. The layers have slightly different compositions because the composition of the water changed a bit as crystallization occurred.
4.2.3 Hydrothermal Minerals

If chemical precipitation occurs at elevated temperatures, we call the process hydrothermal. Travertine and tufa, deposited by hot springs, are examples of hydrothermal deposits. Both are composed of calcite that precipitated from hydrothermal solutions, but tufa is more porous than travertine. The photo shows travertine terrace deposits in Wyoming.
Hydrothermal minerals are also created underground. Occasionally, hot circulating groundwater deposits minerals in sufficient quantity to make a valuable ore deposit. Minerals deposited this way include oxides, sulfides, and some others. Hydrothermal ore deposits vary. In some, ore minerals are concentrated in veins or vugs, in others they are disseminated throughout a body of rock. The table below lists some common hydrothermal ore minerals.
| Minerals Common in Hydrothermal Ore Deposits | ||
| mineral class | mineral or mineral series |
chemical formulas |
| sulfide | pyrite pyrrhotite chalcopyrite galena sphalerite molybdenite |
FeS2 Fe1-xS CuFeS2 Pbs ZnS MoS2 |
| tungstate | wolframite | (Fe,Mn)WO4 |
| oxide | magnetite cassiterite pyrolusite |
Fe3O4 SnO2 MnO2 |
| native elements | gold silver |
Au Ag |
Many spectacular mineral specimens come from hydrothermal deposits. Hydrothermal minerals are often brightly colored because they contain transition metals. Many are metallic and many form highly symmetrical crystals. The photos below show three examples.



4.2.4 Metamorphic Minerals

Metamorphism sometimes involves recrystallization and coarsening of a rock with no change in mineralogy. Often, however, it involves chemical reactions and replacement of preexisting minerals by new ones. Bonds are broken and atoms migrate by solid state diffusion or are transported short distances by intergranular fluids to sites where new minerals crystallize and grow. The photo shows a large red garnet crystal in a highly deformed metamorphic rock called a mylonite. Large minerals of this sort in metamorphic rocks are called porphyroblasts.
The mineralogy of metamorphic rocks is more diverse than in sedimentary or igneous rocks. Nearly all the minerals found in igneous rocks can be present in metamorphic rocks. Many minerals that are found in sedimentary rocks may be present as well. In addition, other minerals, uncommon or nonexistent in igneous and sedimentary rocks, form through metamorphism. The table below lists only a few of the more common metamorphic minerals.
| Common Metamorphic Minerals (besides those common in igneous and sedimentary rocks) |
|
| mineral | chemical formula |
| cordierite tremolite wollastonite andalusite kyanite sillimanite staurolite chloritoid garnet zoisite |
(Mg,Fe)2Al4Si5O18 Ca2Mg5Si8O22(OH)2 CaSiO3 Al2SiO5 Al2SiO5 Al2SiO5 Fe2Al9Si4O23(OH) (Fe,Mg)Al2SiO5(OH)2 (Ca,Fe,Mg)3(Al,Fe)2Si3O12 Ca2Al3Si3O12(OH) |

Metamorphism may involve replacement of one mineral by another. For example, calcite may become aragonite or vice versa. Both minerals are CaCO3, but their atomic structures differ. Mineralogical changes due to metamorphism, however, usually involve several different minerals reacting together. Dolomite (CaMg(CO3)2) and quartz (SiO2) may react to form diopside (CaMgSi2O6) if a limestone containing quartz is metamorphosed at high temperature. The photo here shows green diopside surrounded by dolomite and calcite.
4.2.5 Weathering and Diagenesis

Weathering is the breaking down or decomposition of rocks and minerals because of reactions with the atmosphere or water, and sometimes biological organisms. The process occurs in place at or near Earth’s surface, and is distinct from erosion, which involves transportation of material by water, wind, gravity and other agents. During weathering, large rocks or mineral grains typically break apart. Simultaneously, new minerals may form from old ones. Iron oxides, quartz and clay often dominate highly weathered material. These minerals formed as other minerals disappeared. The photo shows a boulder with a distinct red layer of iron oxide on its outside that formed by weathering.
Diagenesis can occur after weathering has taken place, especially after a sediment is deeply buried. It involves physical and chemical changes in sediments that occur as the sediments are lithified and turn into a sedimentary rock. Diagenesis commonly leads to minerals disappearing and being replaced by others through processes involving both weathering and low-temperature metamorphism. Thus, no sharp boundary exists between diagenesis and metamorphism, although many geologists choose an arbitrary burial temperature of 200 oC as the beginning of metamorphism. Agents of diagenesis include pressure and heat caused by burial. Often, hydrothermal solutions are involved as well.
Chapter 7 includes a more detailed discussion of weathering and diagenesis.
4.3 Mineral Stability and Polymorphs
Of the thousands of known minerals, a relative few are very common. A key reason is that many minerals are only stable under specific conditions. In 1878, J. Willard Gibbs defined a form of energy that determines compound stability. We now call it the Gibbs free energy and indicate it by the variable G. Notions involving Gibbs free energy form the basis for the field of thermodynamics. As pointed out by Gibbs, natural chemical systems are most stable when energy is minimized. So, minerals and mineral assemblages with low Gibbs energy are more stable than those with high energy. Consequently, unstable minerals break down to form different minerals, with lower Gibbs free energy, over time. Thus, minerals with relatively low Gibbs energies are more common than others.
Elements can combine in many ways to form crystals, but as atoms bond, they naturally tend to arrange themselves in the way that minimizes Gibbs free energy. For example, mineralogists have identified more than half a dozen naturally occurring polymorphs of silica (SiO2). Polymorphs are minerals that have identical compositions but different arrangements of atoms and bonds. The polymorph with the lowest chemical energy is the stable form of SiO2. Different polymorphs have the lowest energy under different pressure-temperature conditions. At room temperature and pressure α-quartz has the lowest energy and is stable; the other polymorphs are thermodynamically unstable.
Under normal Earth surface conditions, because α-quartz is stable, we would expect it to form to the exclusion of the others. This is generally the case, but there are exceptions. For kinetic and other factors, natural systems may not always reach lowest (stable) energy conditions. So, especially at low temperatures, we sometimes find examples of metastable SiO2 polymorphs – these are minerals that should, in principle react to become α-quartz if we waited long enough. At elevated temperatures and pressures, metastable α-quartz cannot persist. It changes into other SiO2 minerals (such as cristobalite, coesite, tridymite or stishovite) that have lower chemical energy (Box 4-2).
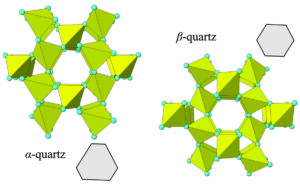
A change from one polymorph to another can be a reconstructive transformation involving major rearrangement of atoms and bonds. Alternatively, it may be a displasive transformation that involves bonds stretching or shrinking (not breaking) and angles between bonds changing, as one mineral turns into another. An example of a displasive transformation is the polymorph change when high-temperature SiO2, β-quartz, turns into α-quartz with cooling. It is very subtle, but, as seen in Figure 4.25, the difference between the two polymorphs is whether the structure contains perfectly hexagonal symmetry and openings. β-quartz does (drawing on the right) and α-quartz does not (drawing on the left). The video below shows the structure of α-quartz. Look closely and you will see that it begins with a view down the channel similar to the photo on the left above in Figure 4.25.
A third kind of transformations is an order-disorder transformation. These involve atoms ordering and arranging in slightly different ways and are gradual changes that occur over a range of pressures or temperatures.
Transformations of any kind may occur quickly or may be very slow. Reconstructive transformations are generally sluggish, and may not occur even if they should. For example, all diamonds should turn into graphite (a reconstructive transformation) at Earth’s surface, but they do not. In contrast, displasive transformations, such as α-quartz turning into β-quartz are instantaneous and reversible. Heat a quartz crystal to just over 573 °C and it changes into β-quartz . Cool the same crystal and it will change back into α-quartz. Order-disorder transformations, the third kind of transformation, occur at different rates depending on how fast temperature, and sometimes pressure, change. If the rate of change is fast, no transformation may occur.
● Box 4-2 Silica Polymorphs Many known minerals have composition SiO2. Some of the most important include quartz, tridymite, cristobalite, coesite, and stishovite. This photo shows grains of coesite; the largest is about 0.4 mm across. The coesite looks a lot like quartz, but has a different internal arrangement of silicon and oxygen atoms. For some spectacular scanning electron microscope images of silica polymorphs, see Figure 12.35 in Chapter 12. Two of the most important polymorphs, α-quartz and β-quartz, are stable at low pressure. α-quartz is by far the most common of the silica polymorphs because it is stable at room temperature and pressure conditions. Because it is stable at lower temperature than β-quartz, α-quartz is sometimes called low quartz, and β-quartz is sometimes called high quartz. At 1 atm pressure, β-quartz exists only at high temperatures. Upon cooling it will turn into α-quartz at 573°C, so we have no room temperature samples of β-quartz to examine. Tridymite and cristobalite are silica polymorphs that, like β-quartz, are found in high-temperature rocks – mostly only in silica-rich volcanic rocks. They are unstable at room temperature and should become α-quartz. However, some samples of these two minerals persist as metastable minerals. For example, the snowflakes that form in volcanic glass (see Figure 4.5) consist of metastable cristobalite crystals. Coesite and stishovite are dense silica polymorphs that form at very high pressure. They are unstable at Earth’s surface. Coesite, which forms at pressures above 25 Kbar (equivalent to 75 km depth in Earth), was first found in meteorite impact craters, later in a few eclogite xenoliths from the mantle, and more recently in what are called ultrahigh-pressure (UHP) crustal rocks. Stishovite forms at even greater pressures (and depths) than coesite. We see it today as microscopic grains in meteorite impact craters and in some rare ultra-high pressure rocks. Whether in impact craters, xenoliths, or UHP rocks, both coesite and stishovite often show signs of reacting to become α-quartz, although the reactions do not always go to completion. |
An important corollary to the laws of thermodynamics, the phase rule, says that the number of stable compounds that can coexist in any chemical system must be small. Thus, not only are stable minerals predictable, they are limited to a small number. For a given rock, the stable minerals may not be the same under all conditions. If a rock is metamorphosed due to pressure or temperature changes, minerals may react to produce new minerals with lower Gibbs free energy. When they stop reacting, they have reached equilibrium. If the Gibbs free energy is minimized, the system is at stable equilibrium.

Consider a chemical system containing Fe-metal and O2. These elements can exist in their pure forms (as metallic iron and gaseous oxygen), but when mixed, they tend to react to produce magnetite (Fe3O4), or hematite (Fe2O3), perhaps creating minerals like the ones seen in the two photos here (Figures 4.27 and 4.28). Both minerals have lower Gibbs free energies than mixtures of Fe and O2. This same principle applies to more complicated systems involving many elements, for example a rock. For any given composition rock, one (stable) mineral assemblage has the lowest energy. If the rock reaches stable equilibrium, the stable assemblage will prevail.

Although the laws of thermodynamics tell us what the most stable mineral(s) should be, they do not tell us how long it will take to reach stable equilibrium. We all know from experience with cars, for example, that it may take a while for the iron in steel to rust, even though (unrusted) iron is unstable at Earth’s surface. The same is true for reactions involving minerals. Some low-temperature systems never reach stable equilibrium, and remain in an intermediate stage called metastable equilibrium when reactions cease. For example, countless numbers of diamonds exist at Earth’s surface, although graphite is a more stable form of carbon. Unless diamonds are heated, they remain metastable and do not change into graphite, no matter how long we wait. In contrast with low-temperature systems such as those at Earth’s surface, most higher-temperature natural systems approach stable equilibrium given enough time.
4.4 Factors Controlling Crystal Size and Perfection
4.4.1 Time and Temperature
The most important factors controlling crystal size and perfection are temperature, time, abundance of necessary elements, and the presence or absence of a flux. All work together, but we can make some generalizations. Temperature is important because at high temperatures atoms are very mobile. Crystals can grow quickly; large and well-formed crystals may be the result. Principles of thermodynamics tell us that crystals that form at high temperatures have simpler atomic structures than those that are stable at low temperatures. This is one reason they can be large and well ordered.
Time is important because if a crystal has a long time to grow, it will naturally be larger and better ordered than one that grows quickly. More atoms will migrate to the growing crystal and order themselves in a regular way. This explains why intrusive igneous rocks, which cool slowly underground, are coarser-grained than extrusive igneous rocks of the same compositions. Some extrusive igneous rocks, such as obsidian, cool so quickly that they contain glass.
Whatever the time and temperature, crystals cannot grow large if the necessary elements are not available. In most rocks, a dozen elements or less account for 90% of the composition. Minerals composed of those elements will usually be larger than those composed of rarer elements. But, even if time, temperature, and atoms are right, crystals may not grow large. Diffusion of atoms through solids is slow, and atoms may not migrate easily to spots where crystals are growing. However, if a hydrothermal fluid is present, it can serve as a flux that delivers atoms to sites of crystallization. For example, the YouTube video (link above in Box 4-3) shows crystals growing from a drop of water – the water delivers atoms to sites of crystal growth.

Magmas, too, can act as fluxes. So, hydrothermal and igneous minerals may grow relatively quickly, and even minerals composed of rare elements may grow to be large. This explains why large crystals of unusual composition may form in pegmatites. Pegmatites form from magmas rich in hydrothermal fluids that concentrate exotic elements like beryllium. The photo shows green beryl, one of the most common beryllium minerals, in a pegmatite from Brazil.
4.4.2 Ostwald Ripening
Whether in magma or aqueous solution, initial crystallization usually involves many nuclei and small crystals. As crystallization continues, however, larger crystals form at the expense of smaller ones. So, regions around large crystals become depleted in small ones. This occurs because nucleation is a kinetic process. Small nuclei composed of just a few atoms form relatively quickly compared with larger crystals. Larger crystals have greater volume-to-surface-area ratio and lower relative surface energy. This means that larger crystals are more chemically stable because molecules in the interior of crystal are less reactive and have lower energy than those on the outside. Consequently, with time, energetics trumps kinetics and molecules on the outside of small crystals diffuse and add to the outside of larger ones. This process is called Ostwald ripening.
Ostwald ripening explains, for example, why ice crystals form over time in initially smooth ice cream – see Figure 4.31 – which makes old ice cream crunchy. The ripening also explains why large crystals (called phenocrysts) surrounded by a sea of small crystals may form in some volcanic rocks. The rock seen in Figure 4.32 contains phenocrysts of black hornblende in a volcanic rock called andesite. The fine-grained material around the hornblende is mostly plagioclase but includes minor K-feldspar, quartz, and magnetite. Note that the hornblende is euhedral because it was surrounded by liquid (melt) as it crystallized and, consequently, atoms could easily migrate to growing crystal faces.
4.4.3 Organized Atomic Arrangements
Because like charges repel and opposite charges attract, as atoms come together to form crystals, cations bond to, and are surrounded by, anions. Anions bond to, and are surrounded by, cations. This occurs even if bonding is not entirely ionic. So, crystal structures generally consist of alternating cations and anions in three dimensions. These charge balance considerations are the reasons that ideal crystals have an ordered atomic arrangement. The distribution of ions is the same in all parts of the crystal and does not depend on crystal size. If more than one arrangement is possible, crystals will naturally grow in the way that minimizes energy.
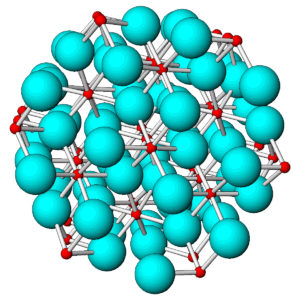
Most common anions such as O2-, are larger than common cations. So, we can think of ionic mineral structures as involving small cations (shown in red) surrounded by larger anions (shown in blue). The number of cations around each anion depends primarily on their relative sizes. In this figure, which is based on the atomic arrangement in corundum, six anions bond to each cation, and four cations bond to each anion. In other minerals, cations and anions may have fewer or a greater number of bonds than this.
4.4.4 Crystal Defects
A hypothetical perfect crystal has an ordered atomic structure with all atoms in the correct places. Yet, as pointed out by C. G. Darwin in 1914, such crystals cannot exist. While a crystal may look perfect on the outside, atomic structures always contain some flaws, called defects.
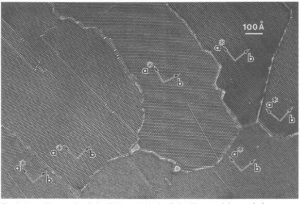
Today, techniques involving X-ray, transmission electron microscope (TEM), and, most recently, high-resolution transmission electron microscope (HRTM) allow crystallographers to look at atomic arrangements and to see relationships between individual atoms. The image in Figure 4.34 shows the atomic structure of crocidolite, an asbestiform amphibole, obtained with a transmission electron microscope. The black and white colors are atomic units composed of a small number of atoms. The entire view shows an imperfect grain composed of multiple subgrains with slightly different atomic orientations (shown by the letters and vectors labeling crystallographic axes – discussed in a later chapter). The apparent offsets in the structure, called zipper faults, are lines along which the atomic structure is defective. In some places, especially along subgrain boundaries, a coarsening of texture suggests small areas that have atomic structure dissimilar from that of normal crocidolite.
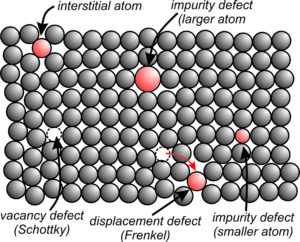
Perhaps the simplest type of defect is an impurity defect, occurring when a foreign atom is present in a mineral’s atomic structure (Figure 4.35). No mineral is perfectly pure. Minerals always contain minor or trace amounts of elements not described by their formula, often at levels that we cannot detect using standard analytical techniques. As seen in this schematic drawing, a larger or smaller atom may replace one normally in the structure, or an atom may occupy an interstitial site. All these examples are types of point defects, so named because they occur at one or a few points in the structure.
Other types of point defects include Schottky and Frenkel defects (both shown in the Figure 4.35. Schottky defects occur when an atom is displaced from a structure altogether, leaving a vacancy or hole. Such defects involve both cations and anions and, to maintain charge balance, missing anions must be matched by missing cations. Frenkel defects occur when an atom is displaced from the position it normally occupies to an interstitial site. Frenkel defects affect both cations and anions, but cation defects are more common because anions are larger and usually more tightly bonded in place.
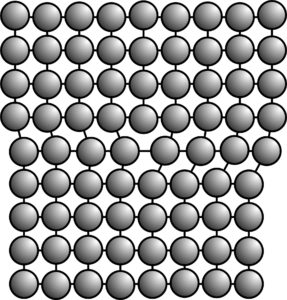
Besides point defects, other types of defects include line defects and plane defects. Line defects, including edge dislocations, like the one shown in the schematic in Figure 4.36, are defects that end at lines in a structure. Plane defects, as their name implies, are planes along which a crystal’s structure is displaced or distorted. On a large scale, grain boundaries are types of plane defects. At the atomic level, several different structures may separate slightly misoriented portions of a crystal structure so that a crystal contains domains having slightly different atomic orientation. Domains of this sort are clearly seen in the TEM photograph above (Figure 4.34).
4.4.5 Crystal Zoning

Crystallizing magmas may produce uneven mineral distribution within a rock. On a smaller scale, individual minerals develop compositional zoning if different parts of a mineral have different compositions. Zoning is present in many minerals but often on such a small scale that we have difficulty detecting it. Sometimes, however, zones of different compositions are large and have different colors – as can be seen in these fluorite crystals from China (Figure 4.37). Look, also, at the zoned tourmaline crystal in Figure 4.13, earlier in this chapter. Often – even if not visible with the naked eye – zoning can be seen with a petrographic microscope because zones of different composition have different optical properties. In still other cases detailed chemical analyses are needed to detect zoning’s presence. Note that, in contrast with the fluorite in Figure 4.37, there is no visible zoning in the fluorite seen in Figure 4.3 (near the start of this chapter).
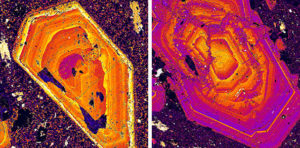
Most zoning is an artifact of crystal growth. It may result from changes in pressure or temperature during crystallization. It may also result from changes in magma or fluid composition as crystals grow. The principles of thermodynamics dictate that zoned minerals are unstable and should homogenize over time. But they are common in nature because diffusion of elements is often not fast enough for growing minerals to remain homogeneous. Most zoning is concentric, forming as growth rings about an original crystal seed. Occasionally, it is more complex and results in compositional zones that are difficult to explain and interpret. The colorful images seen in Figure 4.38 show zoned plagioclase in an igneous rock. These images were obtained with a scanning electron microscope (SEM) and the colors show domains of different compositions that developed as the crystals grew.
4.4.6 Crystal Twinning


In ideal crystals, atoms are in repetitive arrangements, oriented the same way in all parts of the crystal. Twinning result when different domains of a crystal have different atomic orientations. The photo of twinned gypsum on the left (Figure 4.39) is a good example. Half the crystal grew with atoms oriented differently from atoms in the other half. This kind of twinning of gypsum is called swallowtail twinning, for obvious reasons.
Some twinning, called contact twinning, appears as two or more crystal domains in contact with each other (like the gypsum above). The domains share atoms along a common surface, typically a plane called the composition plane. Twins differ from crystal intergrowths composed of crystals that grow next to each other. In a twinned crystal the structure and bonds continue across the composition plane; in intergrowths they are discontinuous.
Another kind of twinning, called penetration twinning, appears as crystals that seem to have grown through each other. In such twins, two domains share a volume of atoms, not just a plane of atoms. The twinned staurolites in Figure 4.40 are good examples of penetration twins. The staurolite specimen includes both a cruciform (cross-like) twin, sometimes called a fairy-cross, and a V-twin that resemble slightly the twinned gypsum on the left. The fluorite crystals in Figure 4.37 also display penetration twins.

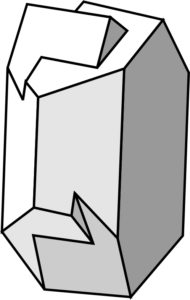
Simple twins comprise only two domains that share common planes or volumes of atoms. The gypsum and staurolite seen above are examples. The twinned orthoclase (K-feldspar) crystals seen here in Figure 4.41 are also examples of simple penetration twinning. The drawing better depicts the nature of the crystal intergrowths.
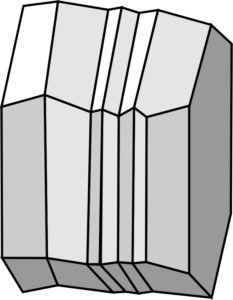

Crystals with complex twins, in contrast with simple twins, have more than two individual twin domains. The drawing and photo (Figures 4.43 and 4.44) show complex twinning called polysynthetic twinning that often characterizes plagioclase, calcite, and a few other minerals. The mineral specimen shows twin striations (stripes) with different reflectivities, because the alternating domains contain atoms arranged in slightly different ways. The presence of striations is one way that geologists distinguish plagioclase from other feldspars, such as the orthoclase seen in Figure 4.41.

Figure 4.45 shows another example of complex twinning called cyclic twinning. The mineral is cerussite (PbCO3). In cyclic twins, three or more crystals seem to emanate from a central point, so the different crystal domains are not parallel but instead are radiating. This photo shows one very important feature. Identifying twins in hand specimens can be difficult, especially in poorly formed crystals. One diagnostic characteristic is the presence of reentrant angles, like the ones seen in this twinned cerrusite. Two crystal faces intersect to form a reentrant angle when they produce an angular concavity that points toward the interior of a crystal instead of the (normal) exterior.
Twinning comes at all scales and may be difficult to detect. Sometimes we can see it with the naked eye, sometimes we can only see it with a microscope, and sometimes we cannot detect it without more sophisticated devices.
Whether twinning is simple or complex, atoms in different twin domains are related by some kind of twin symmetry. For example, the atomic arrangements in two domains may be mirror images of each other. This is the case for all the fine striations in the plagioclase crystal in Figure 4.44 – the alternating domains are reflections of each other. If not mirror images, two twin domains may be related by rotation, such as in Figure 4.45, and there are other ways that domains can be related, too. So, there are many kinds of twins. The nature of a particular kind – whether it is simple or complex and the kind of symmetry involved – define what is called a twin law and allow different kinds of twins to be named. For example, the plagioclase twinning in Figure 4.44 is albite twinning, and the K-feldspar twinning in figure 4.41 is Carlsbad twinning. The domains in albite twinning are related by reflections across a near vertical plane. The two domains in Carlsbad twins are related by a rotation around a near vertical axis in the crystals shown.
Twins form in several ways. They may be growth twins, transformation twins, or deformation twins. Growth twins form when a crystal first grows. Atoms being added to the outside of an already existing crystal may become slightly misplaced so that all subsequent atoms are arranged in a different orientation than in the original crystal domain. Essentially, a new crystal (a new domain) develops adjacent to the original. If a common plane of atoms is oriented correctly for both domains, the result is a contact twin. If a common volume of atoms is oriented correctly for both domains, a penetration twin has been formed. These relationships are most easily visualized by looking at some of the photos and drawings above.
Transformation twins may form when an existing mineral goes through a phase transition to become a different mineral. This involves polymorphs. For example, the feldspar sanidine (KAlSi3O8) forms at high temperature in volcanic rocks. With cooling it may change into orthoclase, a different form of KAlSi3O8, and then microcline, a third form of KAlSi3O8. During the transformations, atoms in different domains of the crystal may become slightly misoriented with respect to other domains. So, twinned orthoclase and microcline crystals may be the result.
The third kind of twins, deformation twins, may develop if a crystal is subjected to stress. Planes or volumes of atoms may become slightly displace, producing domains with different orientations. This kind of twinning is common in calcite, although generally a microscope is needed to see it. Deformation twins are generally not of great importance to mineralogists.
4.5 Life Spans of Minerals
Some rocks and minerals have survived a long test of time. The Acasta gneiss, which formed 4.03 billion years ago, is generally considered the oldest rock on Earth. It contains two kinds of feldspars, quartz, and minor mafic minerals. The oldest known terrestrial mineral grains are detrital zircon crystals in a conglomerate from the Jack Hills of Western Australia. They are 4.40 billion years old and must have eroded from even older rocks. Some minerals in meteorites are older. Mineral grains from the Murchison meteorite, for example, are 7 billion years old – these are the oldest material found on Earth and are older than the Sun.
Deep within Earth, minerals may disappear due to melting, or they may change into new minerals by metamorphism. Occasionally, at Earth’s surface, they may dissolve in water and disappear. The biggest threat to minerals, at least the minerals that we see most often, however, is that most of them are not stable when exposed to air, water, wind, and other elements at Earth surface. They just do not last very long on a geological time scale.
Many minerals commonly occurring in modern sediments and rocks are too unstable to survive in great abundance in older terrestrial rocks. They may have been in those rocks once, but they have changed into different minerals since then. Earth is 4.6 billion years old. Very few examples of the minerals discussed below have existed for more than the last 7% of Earth’s history.


The two photos above show fresh olivine from Hawaii (Figure 4.46), and a single ancient olivine crystal (the green grain in the center of the specimen) from the Austrian Alps (Figure 4.47). Olivine was once abundant in many terrestrial Precambrian (older than 541 million years) mafic rocks, but since the Precambrian Era, most old olivine has been altered by oxygen, carbon dioxide, and water to make serpentine, iron oxides, and magnesite. Because olivine crystallizes in hot and dry magmas, it is unstable under much cooler and wetter Earth surface and near-surface conditions. Because of olivine’s and pyroxene’s tendency to weather rapidly, detrital olivine and pyroxene are largely restricted to relatively young (Cenozoic; younger than 65 million years old) sediments and sedimentary rocks. However, Precambrian olivine and pyroxene occur in Moon rocks and meteorites that have been isolated from oxygen and water.

Other examples of minerals generally absent from older terrestrial rocks include the quartz polymorph tridymite, and aragonite, a high-pressure polymorph of calcite. Tridymite is common in Cenozoic siliceous volcanic rocks, including rhyolites, obsidian, and andesites. However, except in stony meteorites and lunar basalts, the mineral changes to quartz over time and is rarely found in rocks that are older than Cenozoic age. The photo in Figure 4.48 show snowflake tridymite in a volcanic rock in Germany.

Many marine organisms create shells that consist of CaCO3 in the form of aragonite rather than calcite. Figure 4.49 shows an example. Unless aragonite fossils are deeply buried, they will alter to calcite. The oldest known aragonite fossil is from an organic-rich shale of Mississippian age (around 350 million years old). Geologists have only found aragonite fossils that old in three places. One of the rocks is volcanic while the others are black shale and asphaltic limestone. The presence of abundant organic matter in three of the four known rocks with Paleozoic aragonite is probably responsible for the preservation of the aragonite. The organic matter coated the fossils and probably prevented water from reaching them and promoting their conversion to calcite.

Inorganic aragonite sometimes forms on the ceilings or walls of caves or mines, in much the same way that stalactites and other speleothems form. It is, however, unstable, and over time turns into calcite. The change to calcite is slow, and occurs on time scales of 10 to 100 million years.
Some nonmineral materials are unstable and invert to minerals given a chance. Opal and volcanic glass are amorphous materials (although opal has been approved as a mineral name by the International Mineralogical Association). Over time, both weather or alter into more stable crystalline compounds, such as quartz. This explains why obsidian is rarely found in rocks older than the Miocene. The oldest known volcanic glass is in a 70-million-year-old welded tuff. Opal is slightly more stable than obsidian. Reaction rate calculations indicate that opal will entirely convert to quartz in about 180 million years at 20 °C , approximately 4.3 million years at 50 °C, and in only about 47 years at diagenetic temperatures of 200 °C. Not surprisingly, the oldest known opal is of Lower Cretaceous age, about 125 million years old.blank line
blank line
●Figure CreditsUncredited graphics/photos came from the authors and other primary contributors to this book. 4.1 Aquamarine, tourmaline, orthoclase, Géry Parent, Wikimedia Commons |