
Many Different Minerals
|
Mineral Identification . . . Identifying unknown minerals can be easy or very challenging. An experienced mineralogist focuses on one or a few properties that are most diagnostic. Other people may wish to use some sort of systematic approach or key. Many keys can be found with a Google search, but my favorite is at the URL below. If you use it, be sure to answer all questions with yes or no; otherwise you may not get to the correct tables. |
Geologists and mineralogists have described more than 3,000 minerals; most are exceedingly rare, and it is unnecessary and impractical to try to describe them all in this book. The links below in Table 14.1 take you to descriptions of about 180 individual mineral species. You can also use the navigation menu on the right-side of this web page to find different minerals.
The mineral descriptions in this chapter are arranged in order based on the classification scheme presented in Chapter 1; Table 14.2, below, summarizes it. Links in the table take you to different parts of the system. A brief introduction and tabulation of mineral species introduces each of the classes, subclasses, series, or groups.
|
About the photos . . . Most of the photos in this chapter came from Wikimedia Commons or mindat.org. An especially large number are photos originally taken by Robert M. Lavinsky, Géry Parent, James St. John, and Didier Descouens; they deserve special acknowledgment. Credits for all photos except those that originated at the University of North Dakota are listed at the end of the chapter. Many minerals have many different appearances. This chapter includes representative photos, but you should go to Wikipedia, Wikimedia Commons, mindat.org, or simply Google, if you wish to see all the possible varieties. Be warned: professional mineral photographers like to take pictures of spectacular samples. These are not the kind of specimens you can expect to encounter unless you are very lucky or go to a museum, but they are the ones you are most likely to find in photographs on the web. However, the photos in this chapter were selected so they include both mundane and museum-quality samples. |
This chapter contains descriptions of the most common minerals (but many of them are not very common), as well as descriptions of others that have economic importance. Other species are included if they have unique structures or chemistries, or demonstrate principles or properties not well represented by the common or economic minerals. Still others are here if they are useful indicators of geological environments and processes or if they can be used for practical purposes, such as radioactive age determinations.
The information given here is intended for students of mineralogy, so emphasis has been placed on those properties that best aid in practical mineral identification: hand specimen characteristics and, to a lesser extent, occurrences, associations, and optical properties. The mineral descriptions contain only brief discussions of atomic arrangements and crystal chemistry.
1.1 Silicate Class: Framework Silicates
In the sections that follow, we look systematically at the most common minerals that belong to each of the groups listed above. We start with minerals of the Silicate Mineral Class and the Framework Silicate Subclass.
1.1.1 Silica Group Minerals
| Silica Group Minerals quartz SiO2 cristobalite SiO2 tridymite SiO2 coesite SiO2 stishovite SiO2 |
The silica group minerals are framework silicates with composition SiO2 (silica). They belong to the Silicate Mineral Class. Below, we consider the most important of these minerals in more detail; they are listed in the table on the right.
The most common silica mineral is quartz. Besides quartz, other silica polymorphs include cristobalite, tridymite, coesite, and stishovite. The different silica polymorphs vary in the way SiO4 tetrahedra join to form a three-dimensional framework. All except stishovite have structures based on individual SiO4 tetrahedra linked at their vertices by “bridging” oxygen.
Adding complications, quartz, tridymite, and cristobalite have low-temperature (α) and high-temperature (β) polymorphs with slightly different atomic arrangements. Coesite and stishovite do not. Additionally, scientists have synthesized several other SiO2 polymorphs in the laboratory. Although mineralogists have described more than half a dozen silica polymorphs, only common quartz, properly called low quartz, or α-quartz, exists in substantial amounts; it is the second most abundant mineral in Earth’s crust.
Because they have different atomic arrangements, the different SiO2 polymorphs vary in symmetry. For example, low quartz is trigonal, high quartz is hexagonal, low tridymite is orthorhombic, low cristobalite is tetragonal, and coesite is monoclinic.
As discussed in Chapter 6, structural variations among the SiO2 polymorphs reflect the different conditions under which they form. Although low quartz is the only stable SiO2 polymorph under normal Earth surface conditions, some rocks contain metastable stishovite, coesite, cristobalite, or tridymite.
For more general information about silica minerals and their stability fields, consult Chapter 6: Igneous Rocks and Silicate Minerals.
▪Quartz (α-quartz) SiO2
Origin of Name
From German quartz of unknown origin.
Hand Specimen Identification
Hexagonal symmetry, vitreous luster, hardness (H = 7), lack of cleavage, and conchoidal fracture usually serve to identify quartz. It commonly appears clear and translucent, but not always. Quartz is sometimes confused with calcite, beryl, cordierite, or feldspars.




When euhedral, quartz‘s hexagonal prismatic habit can be distinctive. These four photos show examples. You can click on any photo to see an enlarged version.

Figures 14.1 and 14.2 are photos of clusters containing classic clear hexagonal quartz crystals. Crystal clusters of this sort most often occur in geodes or vugs. Figure 14.3 shows a similar cluster that contains amethyst (purple quartz). Figure 14.4 is a photo of a zoned crystal with amethyst at its top and clear quartz at its bottom. Figure 14.5 shows less perfect and “dirtier,” but perhaps more typical, euhedral quartz crystals. The hexagonal symmetry is still clear to see.



The photos above are of euhedral crystals, but most natural quartz crystals are anhedral or subhedral. These three figures show examples. Hexagonal symmetry is not apparent, but vitreous luster, lack of cleavage, and hardness still identify these specimens as quartz.
Physical Properties
| hardness | 7 |
| specific gravity | 2.65 |
| cleavage/fracture | no cleavage or parting; brittle/conchoidal fracture |
| luster/transparency | vitreous/transparent |
| color | most commonly colorless; also white, milky; less commonly purple, pink, yellow, brown, or black |
| streak | white |
Properties in Thin Section
In thin section, quartz is distinguished by low relief, low birefringence (maximum interference colors are gray), lack of color, lack of cleavage, lack of visible twinning, lack of alteration, usually anhedral character, and undulatory extinction. Uniaxial (+); ω = 1.544, ε = 1.553, δ = 0.009.
Crystallography
Low quartz forms trigonal crystals and has a hexagonal unit cell. a = 4.913, c = 5.405, Z = 3; space group $ \small{{R3_1}2}$ or $ \small{{R3_2}2}$; point group $ \small{32}$.

Habit
Quartz may be massive or may form prismatic crystals. Crystals belong to crystal class 32, but appear to have 6-fold symmetry. Common crystals are six-sided prisms terminated by rhombohedrons, sometimes appearing to be hexagonal dipyramids. The quartz crystal in Figure 14.9 is an example (but in the movie Congo, it was misidentified as a diamond). Prism faces often show horizontal growth striations. Rare forms include trapezohedra. Most, if not all, quartz is twinned. Two kinds of twins, Dauphinè and Brazil, are common, but normally cannot be seen with the naked eye.
Structure and Composition
Quartz is always essentially pure SiO2 but may contain trace amounts of other elements. It may be compositionally zoned, as seen above in Figure 14.4. The atomic arrangement consists of a three-dimensional framework of SiO4 tetrahedra, with all oxygens shared by two tetrahedra. At 1 atm, upon heating to 573°C, (1,063°F) minor changes in bond angles cause low quartz (α-quartz) to change into high quartz (β-quartz) with crystal symmetry 622; it inverts to low quartz when cooled.
Occurrence and Associations
Quartz is a common and essential ingredient in many sedimentary, metamorphic, and igneous rocks. It dominates in sandstone and quartzite. It occurs in all silicic metamorphic and igneous rocks. It also dominates in beach sands, many soils, and other sediments.
Varieties
Coarsely crystalline varieties of quartz include citrine (yellow to orange), amethyst (purple), rose quartz (pink), smoky quartz (yellow-brown to black), and milky quartz (milky white). Examples of some of these are in the photos above.
Fibrous microcrystalline varieties of quartz include many types of chalcedony, such as carnelian (red), sard (brown), chrysoprase (apple green), agate (banded or variegated), and onyx (white and gray bands). Jasper (iron red), chert (light gray), and flint (dull dark color) are granular microcrystalline varieties of quartz.


Some specimens of quartz contain inclusions of other minerals. For example, Figure 14.10 is a photo of quartz that contains needles of rutile, and Figure 14.11 shows quartz that contains needles of tourmaline.
Related Minerals
More than a half dozen SiO2 polymorphs exist, but low quartz is the only common one. Opal is an amorphous variety of SiO2 that contains some H2O. Figures 3.28, 7.62, and 9.63 show some examples of opal.
▪Cristobalite SiO2
Origin of Name
Named after occurrence at Cerro San Cristóbal, Mexico.

Hand Specimen Identification
Cristobalite is generally difficult to identify in hand specimen. X-ray or optical techniques are required. Cristobalite is sometimes confused with zeolites if it appears to be filling vugs or openings in basalt.
Occasionally, cristobalite appears as white spheroids or snowflakes in obsidian. Figure 14.12 is a photo of a large block of obsidian containing many white spheroids of white cristobalite. Figure 4.5, from Chapter 4, shows cristobalite snowflakes in obsidian.
Physical Properties
| hardness | 6.5 |
| specific gravity | 2.33 |
| cleavage/fracture | no cleavage or parting; brittle/conchoidal fracture |
| luster/transparency | vitreous/translucent to transparent |
| color | most commonly colorless |
| streak | white |
Properties in Thin Section
Crystal habit, low birefringence, and moderate negative relief characterize cristobalite in thin section. Low cristobalite: Uniaxial (-), ω = 1.489, ε = 1.482, δ = 0.007.
Crystallography
Low cristobalite is tetragonal, a = 4.97, c = 6.93, Z = 4;space group $ \small{{P4_3} {2_1}2}$; point group $ \small{422}$. High cristobalite is cubic, a = 7.13, Z = 8; space group $ \small {F \frac{4_1}{d} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Cubic, octahedral, or coarse aggregates typify cristobalite. Although low cristobalite crystals belong to crystal class 422, they often appear as small octahedra or globby aggregates.
Structure and Composition
Cristobalite, always nearly pure SiO2, may contain minor amounts of Al3+ and alkalis. As with quartz, the structure is based on a three-dimensional framework of SiO4 tetrahedra. The difference between cristobalite, quartz, and other SiO2 polymorphs is the way in which the tetrahedra are linked. Mineralogists have described two cristobalite polymorphs: low cristobalite (α-cristobalite) is tetragonal, high cristobalite (β-cristobalite) is cubic.
Occurrence and Associations
Cristobalite is only found in high-temperature silicic extrusive igneous rocks. Rapid cooling may keep it from changing into the more stable, low quartz. Typically it occurs as small spherical grains or aggregates in vugs, as misty inclusions in volcanic glass, or as principal components in fine-grained ground mass. It is associated with other high-temperature minerals including sanidine and tridymite.
Related Minerals
SiO2 polymorphs include quartz, cristobalite, tridymite, coesite, and stishovite.
▪Tridymite (low tridymite) SiO2
Origin of Name
From Greek for threefold, a reference to its habit of forming compound crystals of three individuals or triangular wedge-shaped crystals.

Hand Specimen Identification
Tridymite is usually sufficiently fine grained that X-ray or optical measurements are needed for identification. It is sometimes confused with zeolites. Samples with grains coarse enough to see easily are rare, but they are distinctive. Figures 14.13 is an example, and Figure 4.48 from a previous chapter shows a different example.
Physical Properties
| hardness | 6 to 7 |
| specific gravity | 2.28 |
| cleavage/fracture | no cleavage/conchoidal fracture |
| luster/transparency | vitreous/translucent to transparent |
| color | most commonly colorless |
| streak | white |
Properties in Thin Section
In thin section, tridymite can be distinguished by its low birefringence, low refractive index (RI), moderate relief, and typical habit often showing wedge-shaped twins. Low tridymite: biaxial (+), α = 1.478, β = 1.479, γ = 1.481, δ = 0.003, 2V = 70°.
Crystallography
Low tridymite is orthorhombic, a = 9.9, b = 17.1, c = 16.3, Z = 64; space group $ \small{P222} $; point group $ \small{ 222} $
Habit
Characteristic habit includes wedge-shaped crystals in vesicles or on the walls of cavities of volcanic rocks. Crystals belong to crystal class 222, but often appear as twinned pseudomorphs after high tridymite (6/m2/m2/m).
Structure and Composition
Tridymite may contain minute amounts of Al3+ and alkalis. Its structure consists of sheets of SiO4 tetrahedra joined together by bridging oxygens. Three tridymite polymorphs are known (low, middle, and high tridymite).
Occurrence and Associations
Tridymite is found in high-temperature silicic igneous rocks, where it commonly associates with other high-temperature minerals, including sanidine and cristobalite. It is also found in some stony meteorites and lunar basalts.
Related Minerals
SiO2 polymorphs include quartz, cristobalite, tridymite, coesite, and stishovite.
▪Coesite SiO2

Origin of Name
Named after chemist Loring Coes, Jr., who first described it in detail.
Hand Specimen Identification
Coesite, only stable at very high pressure, is found in meteor craters and is generally very fine-grained and unidentifiable in hand specimen. Synthetic coesite can be made in the laboratory; the crystals in Figure 14.14 are examples.
Physical Properties
| hardness | 7 to 8 |
| specific gravity | 2.93 |
| cleavage/fracture | none/conchoidal |
| luster/transparency | vitreous/transparent |
| color | colorless |
| streak | white |
Properties in Thin Section
Biaxial (+), α = 1.59, β = 1.60, γ = 1.60 d = 0.01, 2V = 64o
Crystallography
Coesite is monoclinic, a = 7.17, b = 12.33, c = 7.17, β = 120.0o, Z = 16; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Crystals are usually thin tabs, invisible without a microscope.
Structure and Composition
Coesite’s structure consists of a very dense three-dimensional network of SiO4 tetrahedra. In some ways, the structure is similar to that of feldspars.
Occurrence and Associations
Coesite, a rare high-pressure polymorph of SiO2, is known only from meteor impact craters and some rare xenoliths and other rocks of deep crust or mantle origin.
Related Minerals
SiO2 polymorphs include quartz, cristobalite, tridymite, coesite, and stishovite.
▪Stishovite SiO2
Origin of Name
Named after Sergey M. Stishov, an early investigator of high-pressure SiO2 polymorphs.

Hand Specimen Identification
Stishovite only exists as very fine grains and is not readily identifiable. Occurrence in meteor impact craters gives a hint to its identity. Like coesite, stishovite is sometimes synthesized in a laboratory. Figure 14.15 is a photo of synthetic crystals.
Physical Properties
| hardness | 7 to 8 |
| specific gravity | 4.30 |
| cleavage/fracture | {110}/conchoidal |
| luster/transparency | vitreous/transparent |
| color | colorless |
| streak | white |
Properties in Thin Section
Uniaxial (+), ω = 1.799, ε = 1.826, δ = 0.027.
Crystallography
Stishovite is tetragonal, a = 4.18, c = 2.66, Z = 2; space group $ \small{P \frac{4_2}{m} \frac{2_1}{n} \frac{2}{m}} $; point group $ \small{ \frac{4}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Stishovite has a variable habit depending on how it forms.
Structure and Composition
Stishovite is always nearly pure SiO2. The structure is extremely dense and resembles the structure of rutile. In stishovite, Si4+ is in octahedral coordination, in contrast with the other SiO2 polymorphs.
Occurrence and Associations
Stishovite, a rare, high-pressure polymorph of SiO2, is known only from meteor impact craters.
Related Minerals
SiO2 polymorphs include quartz, cristobalite, tridymite, coesite, and stishovite.
1.1.2 Feldspar Group Minerals
 Feldspars are the most abundant minerals in Earth’s crust. Their compositions vary but may be described with the general formula (Ca,Na,K)(Si,Al)4O8. Feldspar structures are based on SiO4 and AlO4 tetrahedra linked to form a three-dimensional framework. They form two series that share one end-member composition: the alkali feldspar series (mainly NaAlSi3O8 –KAlSi3O8) and the plagioclase (mainly NaAlSi3O8– CaAl2Si2O8) series. Figure 14.16 depicts the series and gives specific names for feldspars of different compositions. Feldspars with compositions that plot in the white center region of the triangle are rare or do not exist. Distinguishing among the different kinds of feldspars in a hand specimen can be difficult. All are hard, vitreous, show about the same cleavage (if it shows at all). Alkali feldspars, in particular, have variable colors; plagioclase may also. Plagioclase is generally white, but alkali feldspars may be white, too. Occasionally, feldspars may be confused with other light-colored hard minerals, including spodumene or amblygonite. Feldspars commonly form simple or polysynthetic twins, or combinations. Both contact and penetration twins are possible. Sometimes twinning is visible with the naked eye, but often requires a microscope to be seen. Twin laws include Carlsbad, Baveno, Mannebach, albite, and pericline. For more general information about the feldspar group, see the Section 6.4.2 of Chapter 6. ▪Alkali FeldsparAlkali feldspars range in composition from albite (NaAlSi3O8) to orthoclase (KAlSi3O8). They also contain minor amounts of anorthite (CaAl2Si2O8). Mixing between alkali feldspar and plagioclase is limited, but greatest at high temperature. Figure 14.16, above, shows the typical compositional range for feldspars that equilibrated at normal temperatures. K-rich feldspar may be either of three polymorphs: sanidine, orthoclase, or microcline. The three differ in the way SiO4 and AlO4 tetrahedra are distributed in their structures. Sanidine, the high-temperature polymorph, is most disordered; microcline, the low-temperature polymorph, is most ordered. Orthoclase has intermediate and somewhat variable ordering. Na-rich feldspar, too, has different polymorphs; they include monalbite at high temperature and low-albite at low temperature. Original high-temperature alkali feldspars commonly reequilibrate with cooling. Thus sanidine may reorder to become orthoclase or microcline, and monalbite may change into low albite. Often, however, even if crystals reorder, they maintain their original high-temperature shapes.  At high temperatures, alkali feldspars form complete solid solutions between monalbite (the high-temperature NaAlSi3O8 polymorph) and sanidine (high-temperature KAlSi3O8). Intermediate compositions are often termed anorthoclase. At intermediate and low temperatures, however, anorthoclase is unstable because a solvus limits solid solutions. Consequently, high-temperature alkali feldspars commonly exsolve (unmix) to form two compositions. One composition is KAlSi3O8-rich and the other is NaAlSi3O8-rich. This produces perthite, an exsolved feldspar that contains orthoclase-rich and albite-rich stripes called exsolution lamellae. The specimen in Figure 14.17 is a good example of perthite. The K-rich and Na-rich lamellae have slightly different colors. The Na-rich (albite) lamellae always contain some anorthite component, and thus are equivalent in composition to plagioclase.  Figure 14.17 shows perthite from the Black Hills, South Dakota. The lamellae run vertically in the photo and are distinguished by contrasting shades of white and gray. Figure 14.18 is less typical, but also shows perthite. The specimen contains visible millimeter-thick stringers/lamellae of feldspars with two different compositions. The alkali feldspar lamellae are stained red because alkali feldspar often contains small amounts of hematite. In the sections that follow, we look into the most important alkali feldspar end members in more detail. |
▪Orthoclase KAlSi3O8
Origin of Name
From the Greek word orthos (right angle) and klasis (to break), referring to this mineral’s perpendicular cleavages.

Hand Specimen Identification
The luster, hardness, and color of orthoclase may be similar to other feldspars, but (in contrast with plagioclase) orthoclase is frequently tan, pink, or flesh colored (Figure 14.19); plagioclase is usually white. Orthoclase has cleavage planes that meet at about 90o, like other feldspars. But, orthoclase does not show twin striations like plagioclase does. Association with other felsic minerals also helps identify this mineral.
The photo in Figure 4.41 shows orthoclase that displays classic penetration twins. The combination of tan color with this sort of twinning identifies this mineral. Figures 6.39 (orthoclase from Minas Gerais, Brazil) and 10.61, from previous chapters, show two other examples of orthoclase that have tannish hues.
Distinguishing orthoclase from its polymorphs, microcline and sanidine, can be very difficult without X-ray or optical data. It is sometimes confused with calcite or corundum but can be distinguished by its hardness, which falls between the other two.
Physical Properties
| hardness | 6 |
| specific gravity | 2.56 |
| cleavage/fracture | cleavage angle at about 90o; perfect (001), good (010), poor {110}/uneven |
| luster/transparency | vitreous/transparent/translucent |
| color | colorless, sometimes a bit pink |
| streak | white |
Properties in Thin Section
In thin section, orthoclase has low birefringence, moderate relief, and resemblance to quartz. However, it has negative relief, is biaxial, and is often clouded by fine-grained alteration. It is distinguished from sanidine by 2V and from microcline by its lack of plaid twinning. Biaxial, α = 1.521, β = 1.525, γ = 1.528, δ = 0.007, 2V = 60° to 65°.
Crystallography
Orthoclase is monoclinic, a = 8.56, b = 12.99, c = 7.19, β = 116.01°, Z = 4; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.

Habit
Orthoclase crystals are prismatic, stubby to elongate, and may be flattened or doubly terminated. Penetration twins and contact twins are common. Figure 14.20 shows typical twinned orthoclase.
Structure and Composition
The structure of orthoclase consists of a three-dimensional framework of SiO4 and AlO4 tetrahedra. K+ ions occupy available holes between the tetrahedra. Most orthoclase contains some Na replacing K; complete solid solution between orthoclase and albite (NaAlSi3O8) is possible only at high temperature. Some orthoclase contains small amounts of CaAl replacing NaSi.
Occurrence and Associations
Orthoclase is common in many kinds of silicic igneous rocks, sediments such as arkoses, and a variety of metamorphic rocks. Quartz and micas are typically associated minerals.
Varieties
If orthoclase shows opalescence, we call it moonstone, Figure 14.21 shows an example.
Related Minerals
The principal K-feldspar polymorphs are sanidine (high-temperature form), orthoclase (moderate temperature form), and microcline (low-temperature form). They differ in the way SiO4 and AlO4 tetrahedra are arranged in their structure. Several other related minerals are known: adularia is a colorless, transparent form of K-feldspar that forms prismatic crystals.
▪Sanidine (K,Na)AlSi3O8
Origin of Name
From the Greek word sanis (tablet) and idios (appearance), referring to this mineral’s typical habit.

Hand Specimen Identification
Sanidine may be difficult to tell from other feldspars, but its restricted occurrence in felsic volcanic rocks and association with other high-temperature minerals are helpful diagnostic tools. In contrast with other alkali feldspars, sanidine tends to be colorless, and often transparent to translucent.
Certain identification requires X-ray analysis or thin sections. Plagioclase and microcline have different kinds of twins than sanidine, and sanidine never shows twin lamellae. Most sanidine probably changes into orthoclase or microcline with cooling and time, but it usually retains its original monoclinic crystal shape.
Physical Properties
| hardness | 6 |
| specific gravity | 2.56 |
| cleavage/fracture | cleavage angle at about 90o; perfect (001), good (010) |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless or white most of the time |
| streak | white |
Properties in Thin Section
In thin section, sanidine appears similar to orthoclase but has greater 2V. Carlsbad twins may divide crystals into halves. Manebach and Baveno twins may also be present. Biaxial (-), α = 1.521, β = 1.525, γ = 1.528, δ = 0.007, 2V varies depending on structure.
Crystallography
Sanidine is monoclinic, a = 8.56, b = 13.03, c = 7.17, β = 116.58°, Z = 4; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Sanidine crystals are prismatic, may be tabular or elongate, and often have a square cross section. Carlsbad twins are common.
Structure and Composition
Similar to orthoclase, the structure of sanidine consists of a three-dimensional framework of SiO4 and AlO4 tetrahedra. In sanidine the two kinds of tetrahedra are randomly distributed in the structure, while in orthoclase they are partially ordered. K+ ions occupy available holes between the tetrahedra. Na may replace K; complete solid solution between sanidine (KAlSi3O8) and albite (NaAlSi3O8) is possible at high temperature.
Occurrence and Associations
Sanidine occurs in silicic igneous rocks but is restricted to rocks that have cooled quickly. If cooling is slow, orthoclase will be present instead. Typical occurrences are as phenocrysts in rocks such as trachyte or rhyolite.
Related Minerals
Related minerals include the other KAlSi3O8 polymorphs, orthoclase and microcline, and the plagioclase series.
▪Microcline KAlSi3O8
Origin of Name
From the Greek word micros (small) and klinein (to lean), referring to this mineral’s cleavage angles being close to 90°.



Hand Specimen Identification
Hardness (H = 6), vitreous/pearly luster, feldspar cleavage, habit, and association help identify microcline, but it may be hard to distinguish from other feldspars, especially orthoclase, its polymorph. Because they all share similar properties, X-ray or optical measurements may be needed for certain identification of feldspars, especially alkali feldspars.
Microcline comes in several different colors. The large tan crystals seen in Figure 14.23 are typical, as are the green-colored crystals seen in Figure 14.24. This bluish-green color is a key to identification but is less common than more mundane colors. Microcline with this color is called amazonite. Figure 14.25 shows another example of microcline. The specimen is from Brazil; the photo contains white microcline accompanied by gray translucent quartz.
Physical Properties
| hardness | 6 |
| specific gravity | 2.56 |
| cleavage/fracture | cleavage angle at about 90o; perfect (001), good (010) |
| luster/transparency | pearly, vitreous/translucent to subtranslucent |
| color | colorless or white, green, salmon-pink |
| streak | white |
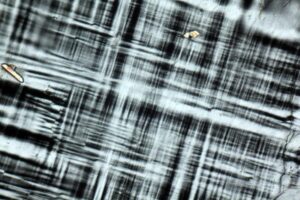
Properties in Thin Section
Microcline’s tartan plaid, also called cross-hatched, twinning (a combination of albite and pericline twinning) is its most diagnostic characteristic in thin section (Figure 14.26). Plagioclase may show two sets of twins at about 90°, but in plagioclase the twins have sharp parallel boundaries, while in microcline they pinch and swell. Microcline is biaxial (-), α = 1.518, β = 1.524, γ = 1.528, δ = 0.010, 2V = 77°-84°.
Crystallography
Microcline is triclinic, a = 8.58, b = 12.96, c = 7.21, α = 89.7°, β = 115.97°, γ = 90.87°, Z = 4; space group $ \small{P1} $; point group $ \small{1}$.
Habit
Prismatic, stubby to elongate, crystals are typical for microcline. It is also commonly found in cleavable masses or irregular grains. Twins, both contact and penetration, may be present but are normally only visible with a microscope.
Structure and Composition
The structure of microcline is similar to the structures of orthoclase and sanidine, but the SiO4 and AlO4 tetrahedra are more regularly ordered, leading to less symmetry. Ordering decreases with increasing temperature of formation: low microcline has complete tetrahedral ordering. Intermediate and high microcline are less well ordered. A solvus limits solid solutions between microcline and albite, NaAlSi3O8, at low temperatures.
Occurrence and Associations
Microcline is common in many kinds of silicic igneous rocks, sediments such as arkoses, and a variety of metamorphic rocks. It easily forms from sanidine or orthoclase as rocks cool from high
temperatures. Quartz and micas are typically associated minerals.

Varieties
When colored green, microcline is given the name amazonite (see Figure 14.27 here and Figure 14.24, above).
Related Minerals
Related minerals include the other KAlSi3O8 polymorphs, orthoclase and microcline, and the plagioclase series.
▪Plagioclase (solid solution feldspar)

Plagioclase feldspars are mostly solid solutions of albite (NaAlSi3O8) and anorthite (CaAl2Si2O8). They commonly contain lesser amounts of orthoclase (KAlSi3O8), especially at high temperatures. See Figure 14.16 At medium and high temperatures, plagioclase may have any composition between albite and anorthite.
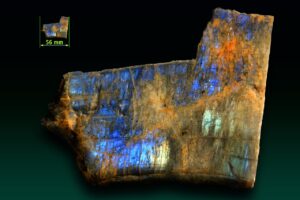
At low temperatures, variable ordering and several small solvi lead to complications not reflected in Figure 14.16. One of the complications is that intermediate-composition plagioclase (called labradorite) may exsolve. This exsolution commonly produces a variety of feldspar that display a play of colors called schiller, or labradorescence. Schiller often looks like colors that may appear on an oil slick. Figure 14.28 shows an example. Figure 6.51 shows additional examples of labradorite.
In the sections that follow, we look at the properties of the two end-member plagioclases, albite and anorthite, but most properties of intermediate compositions are quite similar.
▪Albite NaAlSi3O8
Origin of Name
From the Latin word albus, meaning “white.”


Hand Specimen Identification
Vitreous luster, hardness (H = 6), cleavage angle near 90o, association, and fine polysynthetic twinning help identify albite and other Na-rich plagioclase. If not twinned, it may be difficult to tell from alkali-feldspar. Distinguishing albite from other plagioclase cannot be done precisely without detailed X-ray or optical data.
The albitic feldspar in Figure 14.29 contains fine twin lamellae; they appear as parallel lines that go from the lower-right to the upper left in the photo. The albite-rich plagioclase in Figure 14.30 show two cleavages and has a typical blocky appearance.
Figures 3.63, 6.37, and 10.63 from previous chapters show other photos of albite.
Physical Properties
| hardness | 6 |
| specific gravity | 2.62 |
| cleavage/fracture | cleavage angle at about 90o; perfect (001), good (010), poor {110}/uneven |
| luster/transparency | pearly, vitreous/translucent |
| color | colorless, white, gray, or green |
| streak | white |
Properties in Thin Section
In thin section, albite and other composition plagioclase show no color, have low relief, and exhibit up to 1st-order gray interference colors. Plagioclase may appear similar to K-feldspars and superficially similar to quartz. However, cleavage, biaxial character, and “zebra stripes” caused by polysynthetic twinning usually serve to identify albite and other plagioclase. Biaxial (+), α = 1.527, β = 1.531, γ = 1.538, δ = 0.011, 2V = 77°.
Crystallography
Albite is triclinic, a = 8.14, b = 12.79, c = 7.16, α = 93.17°, β = 115.85°, γ = 87.65°, Z = 4; space group $ \small {P \overline{1}\ } $; point group $ \small {P \overline{1}\ } $.
Habit
Masses or subhedral grains are common for albite and other plagioclase. Rare euhedral crystals are prismatic, tabular, or bladed. Most crystals are twinned according to the pericline law, and some are twinned by the albite law. Albite twins give albite the characteristic polysynthetic twinning that is often visible as fine striations in hand specimen and as stripes in thin section.
Structure and Composition
Albite is an end member of both the plagioclase and the alkali feldspar series. As with K-feldspar, ordering of AlO4 and SiO4 tetrahedra decreases with increasing temperature, leading to minor changes in structure. Low albite’s structure is similar to that of low microcline; high albite’s structure is more disordered. At very high temperature a completely disordered albite, called monalbite because it is monoclinic, is stable. At all but the lowest temperatures, complete solid solution exists between albite and the other plagioclase end member, anorthite, CaAl2Si2O8. Albite, and other plagioclase, also form limited solid solutions with orthoclase, KAlSi3O8.
Occurrence and Associations
The most abundant minerals of Earth’s crust, plagioclase is found in a wide variety of igneous, metamorphic, and, less commonly, sedimentary rocks. Most compositions are intermediate between albite and anorthite, but compositions approaching end members are known. Albite, defined as plagioclase consisting of greater than 90% NaAlSi3O8, is found in silicic igneous rocks such as granite, syenite, trachyte, or rhyolite, where it associates with quartz and orthoclase.

Varieties
Cleavelandite (Figure 14.31) is a form of albite typified by curved plates; it is found in pegmatites. Opalescent varieties of albite or other plagioclase are called moonstone.
Related Minerals
Albite is closely related to the other, more calcic plagioclase, and to alkali feldspars (orthoclase, sanidine, and microcline).
▪Anorthite CaAl2Si2O8
Origin of Name
From the Greek word meaning oblique, in reference to anorthite’s crystal shape.


Hand Specimen Identification
Vitreous luster, hardness (H = 6), cleavage angle near 90o, and association, help identify anorthite, but it can be extremely difficult to tell from other feldspars. Several kinds of twinning are common (see albite habit). Albite twins, if present, may be difficult to see in hand specimen.
Most anorthite-rich feldspars occur in mafic igneous rocks. Figure 14.32 is a photo of basalt with anorthite crystals. It comes from near the summit of Mt. Vesuvius, Italy. Euhedral anorthite crystals like the ones seen in this photo are rare. Figure 14.33 shows a more common occurrence: light-colored bytownite (anorthite containing significant albite component) crystals in a basalt from Cumbria, England. Bytownite is plagioclase that is 70-90% anorthite (see Figure 14.16).
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 2.76 |
| cleavage/fracture | cleavage angle at about 90o; perfect (001), good (010), poor {110}/uneven |
| luster/transparency | pearly, vitreous/translucent |
| color | white or sometimes gray |
| streak | white |
Properties in Thin Section
In thin section, anorthite is similar to other plagioclase. Biaxial (-), α = 1.577, β = 1.585, γ = 1.590, δ = 0.013, 2V = 78°.
Crystallography
Anorthite is triclinic, a = 8.17, b = 12.88, c = 14.16, α = 93.33°, β = 115.60°, γ = 91.22°, Z = 8; space group $ \small {P \overline{1}\ } $; point group $ \small {P \overline{1}\ } $.
Habit
Anorthite is common as cleavable masses or irregular grains. Euhedral crystals are rare. They may be prismatic, tabular, or bladed and are frequently twinned according to the same laws as albite. When present, albite twins give calcic plagioclase characteristic polysynthetic twinning, but the width of the twins is usually greater than is common for albitic plagioclase.
Structure and Composition
Anorthite is the calcic end member of the plagioclase series, but the name is also used for any plagioclase containing > 90% CaAl2Si2O8. Its structure is similar to those of albite and orthoclase. As with the other feldspars, the ordering of AlO4 and SiO4 tetrahedra decreases with increasing temperature, leading to minor changes in structure. Complete solid solution of anorthite with albite, NaAlSi3O8, is possible at all but very low temperatures. Minor solid solution with orthoclase, KAlSi3O8, is common.
Occurrence and Associations
Anorthite, found primarily in mafic igneous rocks, is rarer than other plagioclase. In igneous rocks, it associates with amphibole, pyroxene, or olivine. It is occasionally found in metamorphosed carbonates.
Related Minerals
Anorthite is structurally and compositionally related to the other, more sodic plagioclase, and to the potassic feldspars (orthoclase, sanidine, and microcline).
1.1.3 Feldspathoid Group Minerals
| Feldspathoid Group Minerals analcime NaAlSi2O6•H2O leucite KAlSi2O6 nepheline (Na,K)AlSiO4 |
Feldspathoid minerals are similar to feldspars in many ways, but they contain more Al and less Si. They occur in igneous rocks that contain less SiO2 than normal for the most common igneous rocks.
Like feldspars, feldspathoids have structures based on three-dimensional frameworks of AlO4 and SiO4 tetrahedra; alkalis occupy holes between the tetrahedra. Al:Si ratios vary from 1:1 in nepheline, NaAlSiO4, to 1:4 in the rare feldspathoid petalite, LiAlSi4O10. Feldspathoids are closely related to zeolites; the distinction between the two groups is hazy. The major difference is that zeolite structures contain large cavities or open channels and usually contain molecules of H2O.
The most important feldspathoids are leucite, nepheline, and analcime, although analcime is often considered a zeolite because it contains molecular H2O. Sodalite, Na3Al3Si3O12•NaCl, and haüyne, Na3CaAl3Si3O12(SO4), are sometimes grouped with the feldspathoids but have a cage structure similar to the structures zeolites.
For more general information about the feldspathoid group, see the relevant section in Chapter 6.
▪Analcime NaAlSi2O6•H2O
Origin of Name
From the Greek word analkimos (weak), referring to its weak pyroelectric character.


Hand Specimen Identification
Light color, equant crystals (if euhedral), occurrences in vugs or cavities, and association help identify analcime, but it may be difficult to tell from leucite. Because of its typical crystal shape, it is occasionally confused with garnet, although garnets are generally much more strongly colored. Figure 14.34 shows very large spherical analcime crystals from Martinique, and Figure 14.35 shows similar crystals from Ireland.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 2.26 |
| cleavage/fracture | poor cubic {100}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | color white, gray, pink |
| streak | white |
Properties in Thin Section
In thin section, analcime is colorless and exhibits low negative relief and low birefringence. It may be confused with leucite, which has higher indices of refraction, or with sodalite, which is usually slightly bluish. Isotropic, n = 1.482.
Crystallography
Analcime is cubic, a = 13.71, Z = 16; space group I41/a32/d; point group 4/m32/m.
Habit
Analcime typically forms distinct euhedral crystals. Trapezohedrons and cubes are common forms, often in combination. Massive and granular aggregates are also known. Sometimes crystals appear to be spherical.
Structure and Composition
The framework structure of analcime consists of AlO4 and SiO4 groups joined to make rings of four, six, or eight tetrahedra. Rings align, producing channels that hold H2O molecules. The channels can hold additional absorbed ions or groups. Na+ ions occupy nonchannel sites between rings. Minor substitution of K or Cs for Ca and of Al for Si are common. Analcime forms a limited solid solution with pollucite, (Cs,Na)2(AlSi2O6)2•H2O.
Occurrence and Associations
Analcime is found in cavities in basalt and as a primary mineral in alkalic igneous rocks such as Na-rich basalt or syenite. In cavities, it is associated with zeolites, calcite, or prehnite.
Related Minerals
Analcime is similar in structure to zeolites such as wairakite, Ca(Al2Si4O12)2H2O, and to leucite, KAlSi2O6.
▪Leucite KAlSi2O6
Origin of Name
From the Greek word leukos, meaning “white,” in reference to leucite’s color.


Hand Specimen Identification
Occurrence in Si-poor, K-rich volcanic rocks, light color, crystal habit if pseudocubic, and color help identify leucite. It may be confused with analcime, but leucite typically forms as a matrix mineral whereas analcime forms in cavities.
The most common occurrences of leucite are in alkalic volcanic rocks such as the one seen above in Figure 14.36, above. The white crystals are leucite. Figure 14.37 show rare euhedral crystals of leucite from a classic locality in Brazil.
| hardness | 5.5 to 6 |
| specific gravity | 2.48 |
| cleavage/fracture | indiscernible, poor {100}, poor (001)/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white or sometimes gray |
| streak | white |
Properties in Thin Section
Low relief, gray interference colors, and lamellar or concentric twins help identify leucite. Uniaxial (+), ω = 1.508, ε = 1.509, δ = 0.001.
Crystallography
Leucite is tetragonal, a = 13.04, c = 13.85, Z = 16; space group $ \small{I \frac{4_1}{a}} $; point group $ \small{ \frac{4}{m}} $.
Habit
Although tetragonal at low temperatures, leucite normally has the form of its high-temperature cubic polymorph. Trapezohedral crystals are typical. Polysynthetic twinning may give faces fine striations.
Structure and Composition
Leucite’s structure consists of a framework of 4-, 6-, and 8-membered rings of AlO4 and SiO4 tetrahedra. K+ ions occupy half the available sites between the rings. Leucite is generally close to end-member composition, although small amounts of Fe, Na, and other alkalis may be present.
Occurrence and Associations
Leucite is a rare mineral found in Si-poor, K-rich volcanic rocks. It is never found with quartz.
Related Minerals
Leucite is isostructural with pollucite, (Cs,Na)(AlSi2O6)2•H2O. Chemically, it is closely related to orthoclase, KAlSi3O8; kaliophilite, KAlSiO4; and to analcime, NaAlSi2O6•H2O. A cubic polymorph of leucite exists above 605°C (1,120 °F).
▪Nepheline (Na,K)AlSiO4
Origin of Name
From the Greek word nephele, meaning “cloud,” because crystals turn cloudy when immersed in acid.


Hand Specimen Identification
Occurrence in alkaline igneous rocks, blocky habit, clear or whitish color, lack of cleavage, and greasy luster help identify nepheline. It may be confused with feldspar or quartz but is softer. Occasionally it is confused with apatite. These two photos show typical nepheline of two slightly different colors.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 2.60 |
| cleavage/fracture | indiscernible, poor {100}, poor (001)/subconchoidal |
| luster/transparency | subvitreous to vitreous, greasy/sometimes translucent |
| color | white, colorless, turbid |
| streak | white |
Properties in Thin Section
Low birefringence and relief, and common alteration (to zeolites, sodalite, muscovite, or kaolinite), identify nepheline. It is distinguished from the feldspars by its uniaxial nature and from quartz by its optic sign. Uniaxial (-), ω = 1.540, ε = 1.536, δ = 0.004.
Crystallography
Nepheline is hexagonal, a = 10.01, c = 8.41, Z = 8; space group $ \small{P6_3} $; point group $ \small{6}$.
Habit
Massive, compact, and embedded grains of nepheline are common. Crystals are short and prismatic with six or twelve sides.
Structure and Composition
The structure of nepheline is similar to the structure of tridymite, but every other Si is replaced by Al, and Na occupies large sites between Al and Si tetrahedra. All natural nepheline contains some K substituting for Na, but a solvus exists between nepheline and kalsilite, KAlSiO4, at temperatures below 1,000°C (1,830 °F). Exsolution, similar to perthite (see the general discussion of alkali feldspars, above), is common.
Occurrence and Associations
Nepheline is characteristic of some Si-poor igneous rocks, such as syenite. It is found with feldspars, apatite, cancrinite, sodalite, zircon, and biotite.
Related Minerals
Nepheline is isostructural with tridymite. It has a high-temperature polymorph above 900°C (1,650 °F). It forms a solid solution with kalsilite, KAlSiO4, and is chemically related to kaliophilite, KAlSiO4. Cancrinite, (Na3Ca2)2CO3(Si3Al3O12)•2H2O, is similar to nepheline in many ways and occurs in the same type of rocks.
1.1.4 Scapolite Series Minerals
| Scapolite End Members marialite Na4(AlSi3O8)3Cl meionite Ca4(Al2Si2O8)3(CO3,SO4) |
Scapolite is a solid-solution metamorphic mineral with compositions related to the feldspars, but with structures more closely related to nepheline. The two principal end members are marialite, Na4(AlSi3O8)3Cl, equivalent in composition to albite plus halite; and meionite, Ca4(Al2Si2O8)3(CO3,SO4), equivalent in composition to anorthite plus calcite/anhydrite. Complete solid solution between the two end members is possible; F and OH may replace Cl and CO3.
▪Scapolite (Na,Ca)4(Si,Al)14O24(Cl,CO3,SO4)
Origin of Name
From the Greek word skapos, meaning stalk, in reference to its common woody appearance.


Hand Specimen Identification
Scapolite is characterized by prismatic crystals with square cross sections and two cleavages at 45° to each other. Commonly, euhedral scapolite crystals are translucent like the ones shown in Figure 14.40. Massive samples often have a distinct woody or fibrous appearance, like the specimens seen in the figure. Scapolite may be confused with feldspar when not transparent and gemmy and if its prismatic habit is not evident.
Scapolite is sometimes transparent and gemmy, like the purple scapolite in Figure 14.41.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 2.55 |
| cleavage/fracture | good {100}, poor {110}/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | variable, typically yellow or light brown but also purple, rarely clear |
| streak | white |
Properties in Thin Section
Scapolite may appear similar to feldspars but is uniaxial and has different cleavage. Uniaxial (-), ω = 1.540, ε = 1.536, δ = 0.004.
Crystallography
Scapolite is tetragonal, a = 12.11, c = 7.56, Z = 2; space group $ \small{I \frac{4}{m}} $; point group $ \small{ \frac{4}{m}} $.
Habit
Crystals of scapolite are typically tetragonal prisms, often woody looking and translucent. Massive varieties are common.
Structure and Composition
Scapolite‘s structure is closely related to that of nepheline, consisting of AlO4 and SiO4 joined in a three-dimensional framework. The structure contains two types of holes for anions and anionic groups: one holds Na and Ca; the other Cl or CO3. CaAl substitutes for NaSi freely; SO4 and F may substitute for Cl and CO3. The two dominant scapolite end members are marialite, Na4(AlSi3O8)3Cl, and meionite, Ca4(Al2Si2O8)3(CO3,SO4). Natural scapolite has intermediate compositions. Chemically, scapolite is equivalent to plagioclase plus CaCO3, NaCl, or CaSO4. Minor K, OH, and F may be present.
Occurrence and Associations
Most scapolite occurrences are in calcic metamorphic rocks: marbles, marls or mafic gneisses, and amphibolites. Rare occurrences are reported from igneous rocks. Associated minerals include plagioclase, clinopyroxene, hornblende, apatite, garnet, and titanite. Scapolite easily alters into low-temperature minerals.

Varieties
Scapolites with compositions between meionite and marialite are sometimes called wernerite.
Scapolite gemstones are not abundant, but come from many places around the world. Figure 14.42 shows a rough scapolite crystal, and the faceted and polished gem produced from a similar crystal. There is quite a difference! Typical scapolite gems are either yellow, like the one on the right in Figure 14.42, or purple (see Figure 14.41, above).
1.1.5 Zeolite Group Minerals
|
Zeolite Minerals |
Zeolites are a large and important group of secondary minerals, typically formed by alteration of minerals in igneous rocks. Only five are considered in detail here, but more than 40 natural species are known, and many equivalent phases have been synthesized. Almost all of them have compositions more-or-less equivalent to hydrated feldspars.
All zeolites are framework silicates, containing a three-dimensional network of SiO4 and AlO4 tetrahedra linked to form channels, cages, rings, or loops. Alkalis or alkaline earths occupy large sites in the structures. Some zeolites have 4-, 6-, 8-, or 10-member tetrahedral rings. Others contain complex polyhedra resulting in cage-like openings. Unlike other framework silicates, zeolites contain large open cavities and channels in their structures that permit cations and H2O molecules to pass in and out without disruption. Consequently, zeolites are used as molecular sieves in water softeners and other industrial applications.
Crystal symmetry and morphology vary between species. Different zeolites are cubic, tetragonal, orthorhombic, hexagonal, or monoclinic, and habits may be fibrous, tabular or platy, prismatic, or equant. Although sodalite is sometimes grouped with the feldspathoids, it is here grouped with the zeolites because it has a cage-like structure with 4- and 6-member silica rings.
For more general information about zeolites, consult the zeolites section in Chapter 7.
▪Natrolite Na2Al2Si3O10•2H2O
Origin of Name
From the Greek word natron, meaning “soda.”


Hand Specimen Identification
The many different zeolites have similar occurrences and may be hard to distinguish. Natrolite typically occurs as white radiating needles or as radial aggregates. Its perfect prismatic cleavage and uneven fracture and association also aid identification. Natrolite may occasionally be confused with aragonite or pectolite.
Figure 14.43 is a photo of a mass of natrolite crystals that appear to be radiating from common points. In Figure 14.44, natrolite crystals are separated acicular needles instead of massive, producing a delicate spray.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 2.23 |
| cleavage/fracture | perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless or sometimes gray |
| streak | white |
Properties in Thin Section
Natrolite may be difficult to distinguish from other zeolites. It has two perfect cleavages, has parallel extinction, is length slow, and has a moderate 2V. Biaxial (+), α = 1.48, β = 1.48, γ = 1.49, δ = 0.012, 2V = 38° to 62°.
Crystallography
Natrolite is orthorhombic, a = 18.30, b = 18.63, c = 6.60; space group $ \small{Fd2d} $; point group $ \small{ mm2} $.
Habit
Acicular crystals, often radiating, are typical for natrolite. Crystals may show vertical striations. Radiating rounded masses are also common. Less commonly, it is fibrous, massive, or granular.
Structure and Composition
Natrolite and all zeolites are framework structures built of AlO4 and SiO4 tetrahedra. The tetrahedra are linked to form chains, cages, rings, or loops. In contrast with some other framework silicates, the zeolite structure contains many large holes and channels, which hold weakly bonded H2O. Slightly smaller holes contain Na, Ca, or K. In natrolite, scolecite, and some other zeolites, the tetrahedral framework has a strong linear fabric parallel to the c-axis. Crystal habit is therefore fibrous or acicular. Natrolite always contains minor amounts of Ca and K and has variable H2O content.
Occurrence and Associations
Natrolite (and most other zeolites) are secondary minerals that most commonly form in cracks or on cavity walls in mafic igneous rocks. It also occurs in altered ash or igneous rocks of other kinds, and rarely as a vein mineral. Natrolite is found with calcite and other zeolites.
Related Minerals
Scolecite, CaAl2Si3O10•3H2O, and thomsonite, NaCa2 Al5Si5O20•6H2O, are closely related to natrolite.
▪Chabazite CaAl2Si4O12•6H2O
Origin of Name
From the Greek word chabazios, meaning “hail.”


Hand Specimen Identification
Form, color, and association help identify chabazite but it may be difficult to distinguish from other zeolites. Crystals are usually translucent pseudocubic rhombs, similar to many calcite crystals. Chabazite is, however, distinguished from calcite by its poorer cleavage and lack of reaction to HCl.
Chabazite may have any of a number of different colors. Salmon or orange colors, as seen in Figures 14.45 and 14.46, are most common and help with identification. The pseudocubic shapes of the chabazite crystals are clear in both photos. In Figure 14.45, the chabazite is on top of clear heulandite.
Physical Properties
| hardness | 4 to 5 |
| specific gravity | 2.1 |
| cleavage/fracture | poor rhombohedral {101}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless, salmon, pink, orange, or red |
| streak | white |
Properties in Thin Section
Uniaxial (-), ω = 1.484, ε = 1.481, δ = 0.003.
Crystallography
Chabazite crystals are trigonal. a = 13.17, c = 15.06, Z = 6; space group $ \small {R\overline{3}\frac{2}{m}}$; point group $ \small {\overline{3}\frac{2}{m}}$.

Habit
Chabazite usually forms simple rhombohedral crystals that may, at first glance, appear cubic. Some crystals are more complicated, showing more than one rhombohedral form or exhibiting twinning. Figure 14.47 is a photo of clear chabazite displaying cyclic twinning.
Structure and Composition
Chabazite is similar in structure to natrolite and other zeolites. Tetrahedra form large cage-like openings, which can hold a variety of loosely bonded ions and molecules. The large openings allow diffusion of some small molecules through the structure. Considerable substitution of Na and K for Ca is common.
Occurrence and Associations
Chabazite is a secondary mineral that forms in cracks or on cavity walls in mafic igneous rocks and as an alteration product in silicic igneous rocks. Less commonly, it occurs in sedimentary and metamorphic rocks. It is typically found with calcite and with other zeolites.
Related Minerals
Several other rare zeolites have structures similar to chabazite’s.
▪Heulandite (Ca,Na)2-3(Si,Al)18O36•12H2O
Origin of Name
Named for John Henry Heuland, a British mineralogist.



Hand Specimen Identification
Heulandite is generally platy and tabular. It varies greatly in color and may be difficult to distinguish from other vitreous zeolites, especially clinoptilolite. Crystal habit, perfect one direction of cleavage, and common transparency aid identification.
Figure 14.48 shows clear heulandite crystals from Austria. Figure 14.45, above, shows other clear crystals of heulandite, but with chabazite. Figure 14.49 is a photo of green heulandite from India, and Figure 14.50 shows salmon-colored heulandite in a vug. White/creamy stilbite surrounds the heulandite.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 2.15 |
| cleavage/fracture | one perfect (010)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white, variable, sometimes clear |
| streak | white |
Properties in Thin Section
Heulandite is biaxial (+), α = 1.49, β = 1.50, γ = 1.50, δ = 0.005, 2V = 35°.
Crystallography
Heulandite is monoclinic, a = 17.73, b = 17.82, c = 7.43, β = 116.3°, Z = 4; space group $ \small{Cm} $; point group $ \small{m} $.
Habit
Heulandite crystals are typically platy with a diamond or modified diamond shape, sometimes described as “coffin-shaped.”
Structure and Composition
The structure of heulandite is similar to that of other zeolites, except that tetrahedra are linked in 6-member rings that align to give a more planar structure than most others. End-member calcic heulandite, called mordenite, is rare. Considerable solid solution towards clinoptiloite, a K-containing zeolite, is typical.
Occurrence and Associations
Heulandite is one of the more common zeolites. It is a secondary mineral, found with calcite and with other zeolites, that forms in cracks or on cavity walls in mafic igneous rocks, and in some metamorphic rocks.
Related Minerals
Other zeolites, including clinoptiloite, (Na,K)Si7Al2O18•6H2O, and stilbite, CaAl2Si7O18•7H2O, have structures similar to heulandite’s.
▪Stilbite CaAl2Si7O18•7H2O
Origin of Name
From the Greek word stilbein, meaning “to shine,” referring to this mineral’s luster.



Hand Specimen Identification
Sheaf-like aggregates of twinned crystals, one excellent cleavage, pearly and translucent luster, color, and association identify stilbite.
Figure 14.51 is a photo of typical clear to translucent white stilbite crystals. Figure 14.52 is a photo of salmon-colored crystals, also typical, from India. Figure 14.53 shows creamy white stilbite with blue cavansite (a rare calcium vanadate mineral). The photo in Figure 14.50 shows stilbite that has a similar color.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 2.15 |
| cleavage/fracture | perfect (010)/subconchoidal |
| luster/transparency | pearly, vitreous/translucent |
| color | clear, white, gray, tan, or pinkish |
| streak | gray |
Properties in Thin Section
Cruciform twins and parallel or near-parallel extinction help identify stilbite in thin section. Biaxial (-), α = 1.49, β = 1.50, γ = 1.50, δ = 0.010, 2V = 30° to 50°.
Crystallography
Stilbite is monoclinic, a = 13.64, b = 18.24, c = 11.27, β = 129.16°, Z = 8; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Simple crystals of stilbite are extremely rare. Aggregates of twinned crystals, having the appearance of sheaves of grain, are common. Sometimes aggregates appear fibrous. More rarely, stilbite forms crystals displaying cruciform twinning.
Structure and Composition
Stilbite’s structure is similar to heulandite’s. Considerable substitution of Na and K for Ca is common.
Occurrence and Associations
Stilbite is one of the more common zeolites. It is a secondary mineral, found with calcite and with other zeolites, that forms in cracks or on cavity walls in igneous rocks and in some schists associated with hydrothermal ore bodies.
Related Minerals
Stilbite is grouped with heulandite, CaAl2Si7O18•6H2O, and several others because of its chemical and structural similarity.
▪Sodalite Na3Al3Si3O12•NaCl
Origin of Name
This mineral’s name refers to its sodium content.



Hand Specimen Identification
Color (if blue) and association with other alkalic minerals in alkalic rocks are usually diagnostic for sodalite. If not blue, identification may require chemical tests to tell it from zeolites or analcime. It is sometimes confused with lazulite, another blue mineral, but has different associations.
The photos in Figure 14.54 and 14.55 show typical sodalite samples. Massive anhedral specimens are typical and give little hint of sodalite‘s crystal morphology. The photo in Figure 14.56 shows subhedral crystals of sodalite; subhedral and euhedral crystals of sodalite are uncommon. Sodalite belongs to the cubic crystal system but crystals are generally not cubes.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 2.3 |
| cleavage/fracture | six poor {110} at 60° angles/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | typically blue, rarely whitish |
| streak | white |
Properties in Thin Section
In thin section, sodalite is distinguished by being isotropic and having a low index of refraction and, sometimes, a hexagonal outline. Isotropic, n = 1.485.
Crystallography
Sodalite is cubic, a = 8.87, Z = 2; space group $ \small {P \overline{4}3m} $; point group $ \small {\overline{4}3m} $.
Habit
Often massive or in embedded grains, sodalite forms rare dodecahedral crystals.
Structure and Composition
The structure of sodalite is similar to many zeolites, containing 4- and 6-member tetrahedral rings but, unlike true zeolites, it contains no molecular water that can be driven off easily. The Al and Si tetrahedral rings are linked to form a framework with cage-like openings that hold Cl and sometimes S or SO4. Sodalite is usually close to end-member composition. Small amounts of K or Ca may also be present.
Occurrence and Associations
Sodalite is associated with nepheline, (Na,K)AlSiO4, cancrinite, (Na3Ca2)(Al3Si3O12)CO3•2H2O, leucite, KAlSi2O6, and with feldspars in Si-poor, alkali-rich igneous rocks.
Varieties
Hackmanite is a sulfurous form of sodalite.
Related Minerals
Sodalite is structurally and chemically related to other zeolites and to cancrinite, (Na3Ca2)2(Al3Si3O12)CO3•2H2O.
1.1.6 Beryl and Cordierite
| Other Framework Silicates beryl Be3Al2Si6O18 cordierite (Mg,Fe)2Al4Si5O18 |
Beryl and cordierite, which contain 6-membered tetrahedral rings, are sometimes considered to be ring silicates. They are, however, more properly classified as framework silicates because they have an overall three-dimensional framework of connecting tetrahedra. These two minerals share a common property: open channels in their structures almost always contain some H2O.
▪Beryl Be3Al2Si6O18
Origin of Name
From the Greek word beryllos, meaning “a blue-green gem.”

Hand Specimen Identification
Beryl is often easily identified because of its vitreous luster, typical blue or green color and hexagonal crystals. Occurrence in pegmatites and, less commonly, granites, also aids identification. Even if not clearly hexagonal, color is often a giveaway (Figure 14.57). It is very hard and has poor or no cleavage. If not euhedral or blue, however, it may be difficult to distinguish from other hard vitreous minerals.
Figure 14.58 shows typical blue beryl in a pegmatite. Associated minerals are quartz and orthoclase. Figure 14.59 is also a photo of blue beryl in a pegmatite, but the beryl stands out more clearly. Black tourmaline, orthoclase, and quartz are also present in this specimen. Figure 14.60 is a photo of emerald (green beryl) with quartz. Figure 14.61 is a photo of beryl crystals that have been removed from a pegmatite; they are subhedral to euhedral hexagonal prisms.
Physical Properties
| hardness | 7.5 to 8 |
| specific gravity | 2.7-2.9 |
| cleavage/fracture | poor (001)/even |
| luster/transparency | vitreous/transparent to translucent |
| color | blue, colorless, variable |
| streak | white |
Properties in Thin Section
Beryl is uniaxial (-), ω = 1.568, ε = 1.562, δ = 0.006. In thin section, beryl may appear similar to quartz, apatite, or topaz, but quartz is (+), apatite has higher relief, and topaz is biaxial.
Crystallography
Beryl is hexagonal. a = 9.23, c = 9.19, Z = 2; space group $ \small{P \frac{6}{m} \frac{2}{c} \frac{2}{c}} $; point group $ \small{\frac{6}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Beryl typically forms hexagonal prisms. When present, terminating faces are pinacoids or, more rarely, pyramids. Single crystals are common; columnar aggregates are less so.
Structure and Composition
6-membered tetrahedral rings form sheets that are linked by tetrahedral Be and octahedral Al. Some classification schemes group beryl with true ring silicates such as tourmaline, but in beryl the framework structure is not completely planar and there is much cross linking. Small amounts of Na, Rb, and Li may substitute for Be; minor H2O and CO2 may occupy spaces within the rings.
Occurrence and Associations
Beryl is mostly found in granitic rocks, notably in pegmatites. It may also be found in schists and in rare ore deposits.
Varieties
Beryl can be many different colors due to small amounts of trace elements. Emerald is vivid green; aquamarine is pale blue or greenish blue; morganite is rose colored; heliodor is gold; bixbite is red. The photos below show some of these varieties. The most spectacular examples of beryl are euhedral, like the bixbite, heliodor, and aquamarine examples below. But the emerald specimen is pretty spectacular, too, because of its vivid color.
Related Minerals
Cordierite, (Mg,Fe)2Al4Si5O18, is similar in structure to beryl. Euclase, BeAlSiO4(OH), is another of the rare beryllium silicates.
▪Cordierite (Mg,Fe)2Al4Si5O18
Origin of Name
Named after P. L. A. Cordier (1777–1861), the French mineralogist who first described the mineral.



Hand Specimen Identification
Prismatic cleavage, color, and association aid identification, but if it does not appear similar to dark blue bottle glass, this mineral is often difficult to distinguish without a microscope. It may be easily confused with quartz in hand specimen and with plagioclase in thin section.
Figure 14.66 shows the most common occurrence of blue cordierite – in a high-grade metamorphic rock with red garnet. Figure 14.67 is an enlarged view of a typical blue anhedral cordierite crystal. Cordierite often has a dark gray or black color like the crystals in Figure 14.68. These crystals may show a hint of blue color and have orthorhombic shapes – which helps identify them as cordierite.
Physical Properties
| hardness | 7 to 7.5 |
| specific gravity | 2.5 to 2.8 |
| cleavage/fracture | fair prismatic (010), poor (100)/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | indigo to gray-blue to gray are most common |
| streak | white |
Properties in Thin Section
In thin section, cordierite may be clear or very pale blue-violet. Lamellar twinning is common, similar to plagioclase twinning, except the twins pinch out. Pleochroic halos around zircon inclusions, when present, are easily seen and diagnostic. Cordierite often alters to a fine-grained mass called pinnite. It may be confused with quartz, feldspar, or nepheline. Uniaxial (+ or -), a = 1.54, β = 1.55, γ = 1.56, δ = 0.02, 2V = 65° to 105°.
Crystallography
Cordierite is orthorhombic. a = 17.13, b = 9.80, lc = 9.35, Z = 4; space group $ \small{C \frac{2}{c} \frac{2}{c} \frac{2}{m}} $; point group $ \small{\frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Rare, euhedral crystals are short and prismatic, and may appear pseudohexagonal. Twins are common. Cordierite is most often granular, sometimes massive.
Structure and Composition
Cordierite, like beryl, consists of 6-membered tetrahedral rings joined in a three-dimensional framework. Mg2+, Fe2+, and Al3+ link the rings to each other. Hollow channels, sometimes occupied by H2O or alkalis, run parallel to the c-axis. Fe:Mg ratio is variable.
Occurrence and Associations
Cordierite is found as a product of contact or regional metamorphism in high-grade metamorphosed aluminous rocks. Rare occurrences in igneous rocks have been reported. Associated minerals include garnet, sillimanite, spinel, plagioclase, anthophyllite, and orthopyroxene.
Varieties
A high-temperature polymorph of cordierite, indialite, is isostructural with beryl.
Related Minerals
Cordierite is structurally similar to beryl, and is chemically and structurally similar to osumilite, (K,Na)(Fe,Mg)2(Al,Fe)3(Si,Al)12O30•H2O.
1.2 Silicate Class: Sheet Silicates
1.2.1 Serpentine Group Minerals
| Serpentine Group Minerals antigorite Mg6Si4O10(OH)8 chrysotile Mg6Si4O10(OH)8 lizardite Mg6Si4O10(OH)8 |
Antigorite, chrysotile, and lizardite comprise the serpentine group. Antigorite and lizardite are typically massive and fine grained; chrysotile is fibrous and is one of the few asbestiform minerals. Most of the world’s asbestos is chrysotile; crocidolite and amosite, both amphibole varieties, account for the rest. Chrysotile and lizardite are true polymorphs, but antigorite has a slightly different composition not reflected in its formula.
For more about serpentine polymorphs and occurrences, see the relevant section in Chapter 8.
▪Antigorite Mg6Si4O10(OH)8
Origin of Name
Named after Valle Antigorio, Italy, the first reported place where this mineral was found.
Hand Specimen Identification


Green color, greasy luster, lack of cleavage, and occurrence as a secondary mineral associated with ultramafic igneous rocks identify antigorite. Figures 14.69 and 14.70 contain photos of typical examples. Antigorite crystals are commonly elongate or platy like those seen in these photos. Other occurrences or morphologies may be more difficult to identify. Chrysotile, one of antigorite‘s polymorphs, is a more fibrous variety of serpentine.
Physical Properties
| hardness | 3 to 4 |
| specific gravity | 2.6 |
| cleavage/fracture | perfect (001)/flexible |
| luster/transparency | vitreous to dull or greasy/translucent |
| color | green, yellow-green, gray-green |
| streak | white |
Properties in Thin Section
In thin section, serpentine typically has a net-like pattern and exhibits wavy extinction. Very low birefringence results in anomalous interference colors. It may be confused with chlorite or brucite. Biaxial (-), α = 1.56, β = 1.57, γ = 1.57, δ = 0.007, 2V = 20° to 60°.
Crystallography
Antigorite is monoclinic, a = 5.32, b = 9.50, c = 14.9, β = 101.9°, Z = 4; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Antigorite is commonly fine grained, massive, or platy, in contrast with fibrous chrysotile.
Structure and Composition
The layered structure consists of paired sheets of SiO4 tetrahedra and Mg(O,OH)6 octahedra stacked on top of each other. Antigorite has a slightly different composition from that indicated by its formula because the ratio of brucite layers to tetrahedral layers is slightly greater than 1. Because atoms in the tetrahedral and octahedral layers have slightly mismatched spacings, layers curve slightly. In antigorite the sheets curve in both directions, alternating on a fine scale, so the overall structure retains sheet-like properties. Small amounts of Ni, Mn, Al, Ti, and Fe typically substitute for Mg.
Occurrence and Associations
Antigorite is a common secondary mineral in mafic and ultramafic igneous rocks. It is also found in some marbles. In some serpentinites it is the only mineral present. Associated minerals include magnesium silicates, carbonates, hydroxides, and oxides, as well as olivine, pyroxene, amphibole, magnesite, spinel, chromite, magnetite, brucite, and talc.
Varieties
Garnierite is a Ni-rich variety of serpentine associated with Ni-peridotites.
Related Minerals
Antigorite is closely related to lizardite and chrysotile (common asbestos). Greenalite, Fe3Si2O5(OH)4, the Fe equivalent of serpentine, has a different structure.
▪Chrysotile Mg6Si4O10(OH)8
Origin of Name
From the Greek word chrysos and tilos, meaning “golden” and “fiber.”

Hand Specimen Identification
Fiber-like, asbestiform appearance is diagnostic. Chrysotile may be distinguished from most other fibrous minerals, including fibrous amphiboles, by its greenish-white color. The photo in Figure 14.71 shows a typical example.
Physical Properties
| hardness | 3 to 5 |
| specific gravity | 2.5 to 2.6 |
| cleavage/fracture | none/uneven |
| luster/transparency | greasy, waxy/translucent |
| color | variable white, greenish white |
| streak | white |
Crystallography
Chrysotile is monoclinic, a = 5.34, β = 9.25, c = 14.65, β = 93°, Z = 8; space group $ \small{A\frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
A fibrous, asbestiform habit typifies chrysotile.
Structure and Composition
Composition and structure are similar to those of antigorite, except that the mismatch in spacing of the octahedral and tetrahedral layers causes them to curl in one direction only. The curl around completely and, so, form fibers.
Occurrence and Associations


Occurrence and associations are the same as for antigorite. Chrysotile is a major component of some serpentinites, and it sometimes occurs in veins. Figures 14.72 and 14.73 are photos of veined chrysotile. In many serpentine samples, chrysotile layers are associated with a fine-grained platy polymorph called lizardite. Figure 14.73 shows an example
Related Minerals
Chrysotile has two polymorphs, lizardite and antigorite. Greenalite, Fe3Si2O5(OH)4, the Fe equivalent of serpentine, has a different structure. Amosite and crocidolite, varieties of amphibole, are also sometimes asbestiform.
1.2.2 Clay Minerals
|
Clay Mineral Group |
To a petrologist, the term clay refers to a kind of rock or sediment, usually made of any of a number of minerals referred to as clay minerals. To a mineralogist, clays are a group of sheet silicates with related layered atomic structures. Most are hydrated aluminum or magnesium silicates that form as products of weathering.
Clay minerals fall into three main subgroups that are named after individual species: the smectite, illite, and kaolinite groups. Clays of the smectite group are called expandable clays because they expand when they absorb increasing amounts of water. The most common clay minerals are kaolinite, illite, and montmorillonite. Montmorillonite, a member of the smectite group, is the major component of most bentonite, altered volcanic ash.
Crystal structure and chemistry are variable or poorly determined for many clay varieties. Additionally, some specimens are poorly crystallized and partially amorphous. For these reasons, clay compositions are highly variable and writing precise formulas is often problematic. Clays of the illite and smectite groups are basically three-layer structures (they contain groups of three layers that repeat). Illite group clays, including illite, are transitional from smectites to true micas but lack the alkalis essential to micas.
Kaolinite, which has an atomic arrangement similar to serpentine’s, has a more consistent and ordered atomic arrangement than other clays. It contains a two-layer structure with alternating layers of Al octahedra and Si tetrahedra. Pyrophyllite and talc, sometimes grouped with serpentine, or considered to belong to a separate group, are here grouped with the clays because of similar chemistry. They have, however, three-layer structures more similar to mica structures than to most clays.
For more about clays and occurrences, see Section 7.4.2 in Chapter 7.
▪Montmorillonite – Ca/Na clay of variable composition
Origin of Name
From its original discovery at Montmorillon, near Limoges, France.



Hand Specimen Identification
Montmorillonite and other clays are difficult to tell apart without X-ray study or chemical analysis. When massive, they are unctuous and earthy if wet. They are typically soft, very fine-grained aggregates if dry. The photos in Figures 14.74 and 14.75 show typical examples. Figure 14.76 shows a powdery specimen.
Physical Properties
| hardness | 1 to 1.5 |
| specific gravity | 2.0 to 2.7 |
| cleavage/fracture | perfect {001} rarely visible/ irregular |
| luster/transparency | dull/sometimes translucent |
| color | variable, white or sometimes gray |
| streak | white |
Properties in Thin Section
Montmorillonite’s optical properties are highly variable due to variable chemistry and crystallinity.
Crystallography
Montmorillonite is monoclinic, a = 5.17, b = 8.94, c = 15.20, β = 99.9, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Earthy masses are typical for this mineral.
Structure and Composition
The structure of montmorillonite is based on groups of three layers. Single sheets of (Al,Mg)(O,OH)6 octahedra are sandwiched between two sheets of SiO4 tetrahedra. Montmorillonite is a member of the smectite group (which also includes nontronite and saponite). It forms solid solutions with beidellite, (Na,Ca)Al2(Si,Al)4O10(OH)2•nH2O. Other minor solid solutions are common, and the amount of H2O is variable.
Occurrence and Associations
Clays are secondary minerals, often residual, formed by alteration of Al-rich silicates.
▪Illite – clay mineral with structure and composition related to muscovite
Origin of Name
The name derives from its original discovery in southern Illinois.


Hand Specimen Identification
Illite and other clays are difficult to tell apart without X-ray study or chemical analysis. When massive, they are unctuous and earthy if wet. They are typically soft very fine-grained aggregates that become powdery if dry. Figure 14.77 shows an example.
Figure 14.78 is a photo of a piece of shale made entirely of illite that replaced all other minerals originally present. The square shapes are former halite crystals that are now entirely clay.
Physical Properties
| hardness | 1 to 2 |
| specific gravity | 2.8 |
| cleavage/fracture | perfect {001} rarely visible/ irregular |
| luster/transparency | dull, earthy/sometimes translucent |
| color | variable, gray, white, silvery, or greenish |
| streak | white |
Properties in Thin Section
Illite’s optical properties are variable due to variable chemistry and crystallinity. It is difficult to identify in thin section.
Crystallography
Illite is monoclinic, a = 5.18, b = 8.98, c = 10.32, β = 101.8, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Fine powders or earthy masses are typical for illite.
Structure and Composition
Illite, sometimes called hydromuscovite or hydromica, has structure and composition closely related to muscovite. It contains, however, more H2O and less K than muscovite. It has a mica-like atomic arrangement but is commonly poorly crystallized. Illite may form solid solutions with montmorillonite or with muscovite.
Occurrence and Associations
Illite is a weathering product of muscovite. It is unstable and alters to other clays in a humid environment.

Related Minerals
Glauconite is a green Fe-rich variety of illite that occurs in some sediments and sedimentary rocks. Figure 14.79 shows glauconite in a sandstone. Brammalite is an Na-rich variety of illite, and avalite is a Cr-containing variety.
▪Kaolinite Al2Si2O5(OH)4
Origin of Name
From the Chinese word Kao-ling, the name of the hill that was the first source of kaolinite sent to Europe for ceramics.


Hand Specimen Identification
A white color and clay-like properties, including softness, habit, feel, and earthy smell, help identify kaolinite. Without X-ray data, however, it cannot be distinguished from other white or light-colored clays. These two figures contain photos of typical kaolinite.
Physical Properties
| hardness | 2 to 2.5 |
| specific gravity | 2.6 |
| cleavage/fracture | perfect (001) but rarely seen |
| luster/transparency | dull/sometimes translucent |
| color | white or sometimes very light gray |
| streak | white |
Properties in Thin Section
Optical identification of kaolinite is very difficult. Biaxial (-), α = 1.556, β = 1.563, γ = 1.565, δ = 0.007, 2V = 40°.
Crystallography
Kaolinite is triclinic, a = 5.15, b = 8.92, c = 7.38, α = 91.8°, β = 104.8°, = 90.0°, Z = 2; space group $ \small{P1} $; point group $ \small{1}$.
Habit
Kaolinite is usually massive or a fine-grained aggregate; rare platy, pseudohexagonal crystals have been found.
Structure and Composition
Kaolinite has a two-layer structure: layers of Al(O,OH)6 octahedra alternate with sheets of SiO4 tetrahedra. Several minor substitutions are possible: alkalis or alkaline earths may be present, as well as excess H2O.
Occurrence and Associations
Kaolinite is a common secondary mineral, forming after aluminous silicates. It is a rock-forming mineral, a component of soils, and replaces feldspar in rocks undergoing weathering. Associated minerals include quartz and other minerals resistant to alteration.
Related Minerals
In composition and structure, kaolinite is equivalent to an aluminous serpentine. It has two polymorphs, dickite and nacrite.
▪Pyrophyllite Al2Si4O10(OH)2
Origin of Name
From the Greek word pyro and phyllon, meaning “fire” and “leaf,” in reference to this mineral’s behavior when heated.


Hand Specimen Identification
Softness, somewhat greasy feel, cleavage, and association help identify pyrophyllite . These two photos show classic stellate (radiating star-like habit) pyrophyllite. This texture is diagnostic for pyrophyllite. When not stellate, pyrophyllite cannot be distinguished easily from light-colored clays or related minerals without X-ray data. It may be confused with talc, but talc is softer and has a soapier feel.
Physical Properties
| hardness | 1 to 2 |
| specific gravity | 2.8 |
| cleavage/fracture | perfect basal (001) |
| luster/transparency | pearly/translucent |
| color | white |
| streak | white |
Properties in Thin Section
High birefringence, perfect cleavage, bird’s-eye maple appearance, and lack of color identify pyrophyllite. Talc and muscovite have smaller 2Vs. Biaxial (-), α = 1.553, β = 1.588, γ = 1.600, δ = 0.047, 2V = 52° to 62°.
Crystallography
Pyrophyllite is triclinic, a = 5.16, b = 8.96, c = 9.35, α = 90.03°, β = 100.37°, γ = 89.75°, Z = 2; space group C1; point group 1.
Habit
Individual crystals are unknown. Pyrophyllite is usually massive and foliated, sometimes forming platy or radiating masses like those seen in the photos above.
Structure and Composition
The three-layered structure of pyrophyllite consists of individual sheets of Al(O,OH)6 octahedra sandwiched between sheets of SiO4 tetrahedra. Fe may replace some of the Al; minor Mg, Ca, Na, or K may also be present.
Occurrence and Associations
Pyrophyllite is found in low- and medium-grade metamorphosed shales. Associated minerals typically include kyanite, feldspar, and quartz.
Related Minerals
Pyrophyllite is isostructural with talc, Mg3Si4O10(OH)2, and structurally similar to minnesotaite, Fe3Si4O10(OH)2.
▪Talc Mg3Si4O10(OH)2
Origin of Name
Unknown; perhaps from the Arabic word talq, meaning “pure.”


Hand Specimen Identification
Softness (H = 1) and a greasy feel usually identify talc. It is typically massive with a pearly luster. It is sometimes confused with pyrophyllite, serpentine, or chlorite.
Figure 14.84 shows green pearly talc, a common talc appearance. Figure 14.85 show more typical massive white talc in a talc schist.
Physical Properties
| hardness | 1 |
| specific gravity | 2.8 |
| cleavage/fracture | perfect basal (001)/flexible |
| luster/transparency | resinous, pearly, or silky/translucent |
| color | gray, white |
| streak | white |
Properties in Thin Section
In thin section, talc appears similar to muscovite, chlorite, and pyrophyllite, but has a smaller 2V and often appears smeared or poorly defined when viewed under crossed polars. Biaxial (-), α = 1.54, β = 1.58, γ = 1.58, δ = 0.05, 2V = 6° to 30°.
Crystallography
Talc is monoclinic, a = 5.29, b = 9.10, c = 18.81, β = 100.00°, Z = 4; space group $ \small{Cc} $; point group $ \small{m} $.
Habit
Rare tabular pseudohexagonal crystals have been found, but talc is usually very fine grained and massive.
Structure and Composition
Talc is isostructural with pyrophyllite, being composed of layers of Mg(O,OH)6 octahedra sandwiched between layers of SiO4 tetrahedra. Talc may contain some Ti, Ni, Fe, or Mn but is generally quite pure.
Occurrence and Associations
Talc is a primary mineral in some low-grade metamorphic rocks, including marbles and ultramafic rocks, and less commonly a secondary mineral in mafic igneous rocks.

Varieties
When massive, talc is sometimes called steatite or soapstone. Figure 14.86 shows three examples.
Related Minerals
Talc is isostructural with pyrophyllite, Al2Si4O10(OH)2, and structurally similar to minnesotaite, Fe3Si4O10(OH)2.
1.2.3 Micas
|
Mica Group Minerals Biotite Series Other Micas |
The mica group consists of a number of minerals, the principal ones being biotite and muscovite. All micas are flexible sheet silicates and, when coarse enough, display excellent basal cleavage, a vitreous luster, and are translucent.
The name biotite refers to a series dominated by end members phlogopite and annite. Mg-rich biotite (phlogopite) and Fe-rich biotite (annite) are often difficult to distinguish without detailed X-ray or chemical analyses. In common use, mineralogists often call any black mica biotite and reserve the term phlogopite for brown micas. Color is not, however, a reliable way to distinguish the two.
Muscovite, sometimes referred to as a white mica, is in sharp contrast to biotite. However, it may be confused with margarite, lepidolite, and some other rarer micas that have a light or silvery color. Margarite is one of the brittle micas, a group that includes rarer clintonite and xanthophyllite. Lepidolite is an Li-rich mica found in pegmatites. Although annite and phlogopite enjoy complete solid solution, only limited solution is possible among other end members.
For more general information about the mica group, see Section 6.4.4 in Chapter 6.
▪Phlogopite KMg3(AlSi3O10)(OH)2
Origin of Name
From the Greek word phlogopos, meaning “fiery,” in reference to this mineral’s brown color.


Hand Specimen Identification
Phlogopite, end-member Mg-biotite, is usually identified by micaceous cleavage, brown color, and association. Sometimes euhedral crystals have a pseudohexagonal shape. If not brown, phlogopite is indistinguishable from other composition biotite without additional analytical data.
Figure 14.87 shows a very large pseudohexagonal book of phlogopite from a pegmatite near Bancroft, Ontario. Figure 14.88 is a photo of brown flakes of phlogopite, surrounded by calcite, in a marble.
Physical Properties
| hardness | 2.5 to 3 |
| specific gravity | 2.8 |
| cleavage/fracture | perfect basal (001)/elastic |
| luster/transparency | pearly/transparent |
| color | yellow-brown to brown |
| streak | white |
Properties in Thin Section
Phlogopite is similar to other micas in thin section. It has a smaller 2V than muscovite and is often colored light brown. It may be pleochroic, but not as much as Fe-rich biotite. Biaxial (-), α = 1.56, β = 1.60, γ = 1.60, δ = 0.04, 2V = 0° to 20°.
Crystallography
Phlogopite is monoclinic, a = 5.31, b = 9.19, c = 10.15, β = 95.18°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Coarse books of pseudohexagonal crystals are distinctive but uncommon. More frequently, phlogopite is disseminated as irregular grains or flakes or foliated masses.
Structure and Composition
The basic phlogopite structure is similar to the structures of talc and pyrophyllite: two tetrahedral SiO4 layers surround an octahedral Mg(O,OH)6 layer. Unlike talc and pyrophyllite, however, the three-layer sandwiches are linked by K+ ions occupying large sites between them. Fe often substitutes for Mg, leading to complete solid solution with annite, KFe3(AlSi3O10)(OH)2. Al substitutes for both Mg and Si, creating solid solutions with siderophilite, K(Fe,Al)3(Si,Al)4 O10(OH)2. Limited solid solution with muscovite, KAl2(AlSi3O10)(OH)2, is also possible. Mn, Ti, and a number of alkalis and alkaline earths may also be present, and F may replace some OH.
Occurrence and Associations
Phlogopite is a common mineral in marbles where it associates with calcite, dolomite, quartz, diopside, and tremolite; less commonly, it is found in highly magnesium-rich igneous rocks or in pegmatites.
Related Minerals
Phlogopite has several different polymorphs; they are difficult to tell apart without detailed X-ray studies. It is isostructural with muscovite, KAl2(AlSi3O10)(OH)2, and isotypical with other micas, forming complete or limited solutions with most.
▪Biotite K(Mg,Fe)3(AlSi3O10)(OH)2
Origin of Name
Named after J. B. Biot (1774–1862), a French scientist who conducted detailed studies of micas.


Hand Specimen Identification
Biotite is the name given to a series of dark-colored micas that range from phlogopite (Mg-biotite) to annite (Fe-biotite). Micaceous cleavage, vitreous luster, and sometimes pseudohexagonal crystals are keys to identification. The biotite flakes in Figures 14.89 and 14.90 are typical. The pseudohexagonal biotite crystal in Figure 14.90 is on top of pink calcite.
Biotite is most commonly black but may be brown or tan or, less commonly, other colors. Biotite may be confused with other micas, especially chlorite. Composition is variable and cannot be determined without analysis.
Physical Properties
| hardness | 2.5 to 3 |
| specific gravity | 2.9 – 3.1 |
| cleavage/fracture | perfect basal {001}/ragged |
| luster/transparency | vitreous/transparent to opaque |
| color | black, greenish black, brown-black |
| streak | white |
Properties in Thin Section
Brown, red, or green in thin section, biotite exhibits strong pleochroism, perfect cleavage, bird’s-eye extinction, and parallel or near-parallel extinction. Biaxial (-), α = 1.57, β = 1.60, γ = 1.61, δ = 0.04, 2V = 0° to 32°. Stilpnomelane, a rarer related sheet silicate, looks much like biotite but lacks “bird’s eye” extinction.
Crystallography
Biotite is monoclinic, pseudohexagonal, a = 5.33, β = 9.31, c = 10.16, b = 99.3°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Foliated books of pseudohexagonal crystals of biotite are distinctive but uncommon. More frequently, biotite is disseminated as irregular grains or flakes or foliated masses.
Structure and Composition
The basic biotite structure is identical to that of phlogopite: two tetrahedral layers surround an octahedral layer. The three-layer sandwiches are linked by K+ ions occupying large sites between them. Fe mixes freely with Mg in octahedral sites, leading to complete solid solution between the two principal biotite end members: annite, KFe3(AlSi3O10)(OH)2, and phlogopite, KMg3(AlSi3O10)(OH)2. Coupled substitution of Al for both Mg and Si creates limited solid solutions with siderophilite, K(Fe,Al)3(Si,Al)4 O10(OH)2. Minor solid solution with muscovite, KAl2(AlSi3O10)(OH)2, is also possible: two Al replace three (Fe,Mg), leaving every third octahedral site vacant. Mn, Ti, and a number of alkalis and alkaline earths may also be present, and F may replace some OH.
Occurrence and Associations
Biotite is common in a wide variety of igneous and metamorphic rocks and in immature sediments. Associated minerals include other micas, amphiboles, quartz, and feldspars.
Varieties
Annite and phlogopite are names given to end-member Fe- and Mg-biotite. Siderophilite is the name of biotite that contains excess Al.
Related Minerals
Biotite is isostructural or isotypical with other micas, and similar in many ways to other sheet silicates. Several biotite polymorphs differ in the ways the tetrahedral and octahedral sheets are stacked.
▪Muscovite KAl2(AlSi3O10)(OH)2
Origin of Name
Named after the Muscovy Principality of thirteenth-century Russia, which produced muscovite for use in window panes.


Hand Specimen Identification
Muscovite is the most common mineral of the mica family. Micaceous character and silver color distinguish this mineral. It may be confused with other white micas, such as paragonite or margarite. Certain identification requires optical, X-ray, or chemical analysis. Figures 14.91 and 14.92 contain photos of muscovite from Brazil.
Physical Properties
| hardness | 2 to 2.5 |
| specific gravity | 2.8 |
| cleavage/fracture | perfect basal (001)/elastic |
| luster/transparency | sometimes pearly, vitreous/translucent |
| color | silvery white, sometimes gray-brown |
| streak | white |
Properties in Thin Section
In thin section, muscovite appears clear, has moderate to high birefringence, sometimes has bird’s-eye extinction, a high index of refraction, and one perfect cleavage. It may be confused with colorless phlogopite or with other white micas. Biaxial (-), α = 1.565, β = 1.596, γ = 1.600, δ = 0.035, 2V = 30° to 40°.
Crystallography
Muscovite is monoclinic, a = 5.19, b = 9.04, c = 20.08, β = 95.5°, Z = 4; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Individual crystals are rare. Muscovite typically forms books of mica, often in massive aggregates, with or without a pseudohexagonal outline, or is found as disseminated grains within a quartz–feldspar matrix. Penetration twins may be present.
Structure and Composition
The muscovite structure is identical to that of biotite except that Al has replaced two out of every three (Fe,Mg). Very limited solid solution with annite, KFe3(AlSi3O10)(OH)2, and with phlogopite, KMg3(AlSi3O10)(OH)2, are possible. Na may be present, resulting in solid solution toward paragonite, NaAl2(AlSi3O10)(OH)2. Other alkalis and alkaline earths may also replace K. Li may replace some Al, and F may replace some OH.
Occurrence and Associations
Muscovite is found in many silicic to intermediate igneous rocks, in a wide variety of metamorphic rocks, and in some immature sediments. Associated minerals include other micas, K-feldspar, and quartz.
Varieties
Sericite is a fine-grained form of muscovite created by alteration of feldspars and other alkali-aluminum silicates.
Related Minerals
Muscovite is isotypical with biotite and other micas, such as lepidolite, K(Li,Al)2-3(AlSi3O10)(OH)2. It shares many properties with paragonite, NaAl2(AlSi3O10)(OH)2, and with margarite, CaAl2(Al2Si2O10)(OH)2. Several different muscovite polymorphs are known.
▪Margarite CaAl2(Al2Si2O10)(OH)2
Origin of Name
From the Greek word margarites, meaning “pearl,” a reference to margarite’s color and luster.

Hand Specimen Identification
Margarite is characterized by its micaceous nature, brittleness, and pearly luster. Association and brittleness usually distinguish it from other white micas. Some specimens have a distinctive silvery-pink color. Figure 14.93 is a photo of pink margarite on top of green chlorite. It is from a classic collecting site in western Massachusetts.
Physical Properties
| hardness | 3.5 to 5 |
| specific gravity | 3.1 |
| cleavage/fracture | perfect basal (001)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | gray-yellow, pinkish silver |
| streak | white |
Properties in Thin Section
Margarite is colorless in thin section, resembling muscovite and other white micas. It is distinguished by a higher index of refraction, lower birefringence, and a 6° to 8° extinction angle. Biaxial (-), α = 1.635, β = 1.645, γ = 1.648, d = 0.013, 2V = 45o.
Crystallography
Margarite is monoclinic, a = 5.14, b = 9.00, c = 19.81, β = 108o, Z = 4; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Margarite is typically found in massive, micaceous books and aggregates; individual crystals are rare.
Structure and Composition
Margarite has a structure transitional between muscovite and chlorite. Because the interlayer site is occupied by Ca2+ rather than K+ (as in most other micas), bonds between layers are stronger. Thus, margarite is more brittle (and so is called brittle mica). Minor Na+ and K+ and may replace Ca2+; charge balance is maintained by replacement of Al ions by Be, Ba, Sr, K, Mn, Fe, and Mg ions. Excess OH may also be present.
Occurrence and Associations
Margarite, typically found with corundum and diaspore, is most commonly an alteration product of corundum.
Related Minerals
Margarite is a member of the brittle mica group. Other members of the group include clintonite and xanthophyllite, both having compositions Ca(Mg,Al)2-3(Al2Si2O10)(OH)2. Margarite also shares similarities with stilpnomelane, (K,Ca,Na)(Fe,Mg,Al)8(Si,Al)12(O,OH)36·nH2O.
▪Lepidolite K(Li,Al)(AlSi )(OH)2
Origin of Name
From the Greek word lepid, meaning “scale,” referring to this mineral’s usual habit.


Hand Specimen Identification
Micaceous habit, often a distinctive lilac-gray or rose color (as in Figure 14.94), and association usually serve to identify lepidolite. It sometimes comes in other colors, making identification problematic. For example, Figure 14.95 shows a sample containing small crystals of yellow lepidolite.
Physical Properties
| hardness | 2.5 to 4 |
| specific gravity | 2.9 |
| cleavage/fracture | perfect basal {001}/uneven |
| luster/transparency | pearly, vitreous/translucent |
| color | lilac to rose-red; less commonly, yellow, gray, white |
| streak | white |
Properties in Thin Section
Lepidolite is generally colorless in thin section. It resembles muscovite but has lower relief and lower birefringence. Biaxial, α = 1.53 to 1.55, β = 1.55 to1.59, γ = 1.55 to 1.59, δ = 0.02 to 0.04, 2V = 0o to 60o.
Crystallography
Lepidolite is monoclinic, a = 5.211, b = 8.97, c = 20.16, β = 100.8o, Z = 4; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Lepidolite usually forms coarse- to fine-grained scaly aggregates. It is less commonly disseminated as fine flakes or coarser crystals in pegmatites.
Structure and Composition
Lepidolite is a complex solid solution series with a structure similar to muscovite. Chemically, it is equivalent to muscovite with half or more of the octahedral Al replaced by Li and, perhaps, Fe or Mg. In addition, other alkalis may substitute for K, and O or F may replace OH.
Occurrence and Associations
Lepidolite is restricted to Li-rich pegmatites. Associated minerals include other Li-bearing minerals such as tourmaline, amblygonite, and spodumene, as well as the more common muscovite, feldspar, and quartz.
Related Minerals
Lepidolite has several polymorphs that cannot be differentiated without detailed X-ray study. It is isotypical with other micas.
1.2.4 Chlorite Mineral Group
| Chlorite End Members clinochlore (Mg5Al)(AlSi3)O10(OH)8 chamosite (Fe5Al)(AlSi3)O10(OH)8 sudoite Mg2(Al,Fe)3(AlSi3O)10(OH)8 nimite (Ni5Al)(AlSi3)O10(OH)8 pennantite (Mn,Al)(Si,Al)4O10(OH)8 |
Chlorite is the general name given to a number of Mg- or Fe-rich sheet silicates with similar chemistry and structure. Other sheet silicates with the same atomic arrangement are included in the chlorite group, which contains more than 15 different species.
Common Mg-Fe chlorite compositions vary widely, and they may contain variable amounts of Al. The complex chemical variations and similarity of all Fe-Mg chlorites make identifying individual species problematic, but a few ideal end-member compositions have been given names. Some examples are listed in the blue box. The most common chlorites have compositions close to end-member clinochlore, Mg5Al(AlSi3)O10(OH)8, described below.
Structurally, all chlorites consist of alternating talc-like and brucite-like layers. In both kinds of layers, Fe and Al may substitute for Mg; other elements, such as Ni, may also be present. Composition cannot be determined without X-ray or chemical data, and occasionally with careful optical studies.
▪Chlorite (clinochlore) (Mg5Al)(AlSi3O10)(OH)8
Origin of Name
From the Greek words klinein, meaning “to incline”, referring to the mineral’s inclined optic axes, and chloros, meaning “green.”


Hand Specimen Identification
When fine-grained, a deep green color and occurrence as a replacement mineral generally identify chlorite. The many individual species and varieties cannot be distinguished easily. Chlorite is sometimes confused with talc.
In metamorphic rocks, chlorite may be very fine grained. Figure 14.96 shows the most typical occurrence of chlorite, in a chlorite schist that is so fine grained that individual crystals cannot be seen. The green color, however, reveals the mineral’s presence. If crystals are visible (uncommon) in hand specimen, green color, pseudohexagonal shape, and micaceous habit and cleavage are usually adequate to identify chlorite. Figure 14.97 shows an example of a chlorite crystal.
Physical Properties
| hardness | 2 to 2.5 |
| specific gravity | 3.0 |
| cleavage/fracture | perfect basal (001)/flexible |
| luster/transparency | vitreous/transparent to translucent |
| color | green, variable |
| streak | white or light green |
Properties in Thin Section
Chlorite is generally green and sometimes green-brown in thin section. It usually exhibits yellow-green-brown pleochroism and has moderate to moderately high relief. Birefringence is very low. Interference colors normally are between anomalous blue, brown, or purple and first-order yellow. Biaxial (-), α = 1.56 to 1.60 , β = 1.57 to 1.61, γ = 1.58 to 1.61, δ = 0.006 to 0.020, 2V = 0° to 40°.
Crystallography
Chlorite is monoclinic, a = 5.37, b = 9.30, c = 14.25, β = 97.4°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
When crystals can be seen, chlorite has habits similar to the other micas: foliated books, scaly aggregates, and individual flakes in a quartz–feldspar matrix are typical. Rare pseudohexagonal crystals are known.
Structure and Composition
The structure of chlorite consists of stacked talc, Mg3Si4O10(OH)2 and brucite, Mg(OH)2, layers. The stacking order is variable, leading to a great deal of variety. In both kinds of layers, Al replaces some of the Mg, and in most chlorites Fe is present as well. A general formula, (Mg,Fe,Al)(Si,Al)4O10(OH)8, does not describe all of the compositional variability.
Occurrence and Associations
Chlorite is a common mineral in low- to intermediate-grade metamorphic rocks, diagnostic of the greenschist facies. It is also a common secondary mineral after biotite, muscovite, and many mafic silicates in igneous and metamorphic rocks, and is sometimes found in sediments. Many greenish rocks owe their color to the presence of chlorite. Associated minerals include quartz and feldspars, epidote, muscovite, actinolite, albite, and a number of ferromagnesian silicates.
Varieties
Names given to some idealized chlorite compositions include clinochlore, (Mg5Al)(AlSi3O10)(OH)8; chamosite, (Fe5Al)(AlSi3O10)(OH)8; nimite, (Ni5Al)(AlSi3O10)(OH)8; and pennanite, (Mn,Al)6(Si,Al)4O10(OH)8.
Related Minerals
Chlorite has many varieties and polymorphs. Other similar minerals include cookeite, LiAl4(AlSi3O10)(OH)8; sudoite, Mg2(Al,Fe3)(AlSi3O10)(OH)8; and a number of other hydrated aluminosilicates.
1.2.5 Other Sheet Silicates
| Other Sheet Silicates prehnite Ca2Al(AlSi3O10)(OH)2 apophyllite KCa4Si8O20F•8H2O sepiolite Mg4Si6O15(OH)2•6H2O chrysocolla Cu4Si4O10H4(OH)8•nH2O glauconite ~(K,Na)(Fe,Mg,Al)2(Si,Al)4O10(OH)2 |
A number of other minerals are sheet silicates, or closely related to sheet silicates, but do not fit into any of the groups previously discussed. These include prehnite, apophyllite, sepiolite, chrysocolla, and glauconite. Prehnite and apophyllite, secondary minerals similar in occurrence and association to zeolites are discussed below.
Due to highly variable properties, chemistry, and structure, sepiolite, chrysocolla, and glauconite are not discussed in detail in this book. Glauconite and sepiolite are both clay-like minerals, and chrysocolla is a secondary copper mineral of highly variable chemistry. Okenite, a mineral closely related to zeolites and the others listed in this box, contains both chains and sheets of silicon tetrahedra and so does not fit conveniently into either class.
▪Prehnite Ca2Al(AlSi3O10)(OH)2
Origin of Name
Named after H. van Prehn, who discovered this mineral in 1774.


Hand Specimen Identification
Prehnite, although not a zeolite, occurs in many of the same places that zeolites occur. When green and botryoidal (typical) it is easily identified. Otherwise it may be difficult to distinguish from zeolites, and may also be confused with hemimorphite and smithsonite.
The photo in Figure 14.98 shows a classic example of green botryoidal prehnite. The photo in Figure 14.99 shows spheres of green prehnite with clear calcite.
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 2.9 |
| cleavage/fracture | good basal (001)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | pale green |
| streak | white |
Properties in Thin Section
Prehnite is colorless in thin section. It has moderate birefringence but often exhibits anomalous interference figures, sometimes with an “hourglass” or “bow tie” structure. It may be confused with lawsonite, pumpellyite, epidote, datolite, and a number of zeolites but has higher birefringence than all of them. Biaxial (+), α = 1.625 , β = 1.635, γ = 1.655, δ = 0.03, 2V = 65° to 70°.
Crystallography
Prehnite is orthorhombic, a = 4.65, b = 5.48, c = 18.49, Z = 2; space group $ \small{P2cm} $; point group $ \small{ mm2} $.
Habit
Botryoidal, globular, barrel-shaped, and reniform aggregates are typical. Rarely, prehnite is found as individual tabular or prismatic crystals.
Structure and Composition
Prehnite consists of tetrahedral sheets connected by octahedra. Fe may replace some of the Al.
Occurrence and Associations
Prehnite may be a product of low-grade metamorphism but is more commonly a secondary mineral that forms as crusts or fillings in basalt and other mafic igneous rocks. Associated minerals include pumpellyite, zeolites, datolite, pectolite, and calcite.
Related Minerals
Prehnite is sometimes grouped with the zeolites because of its similar occurrences. Its structure, however, is significantly different.
▪Apophyllite KCa4Si8O20F•8H2O
Origin of Name
From the Greek words for “form” and “leaf,” because it becomes flaky on heating.


Hand Specimen Identification
Like prehnite, apophyllite often occurs where zeolites occur. Tetragonal prismatic crystals, often with terminating pyramid faces, a vitreous luster, and perfect one direction of cleavage perpendicular to prism faces may identify this mineral (especially if it is clear and uncolored). The clear crystals in Figure 14.100 are classic examples.
Figure 14.101 shows a green variety of apophyllite on top of stilbite, a common zeolite. The crystals in both photos have similar shapes, and traces of apophyllite’s one perfect cleavage can be seen in both photos.
Physical Properties
| hardness | 4.5 to 5 |
| specific gravity | 2.3 |
| cleavage/fracture | one perfect (001), poor {110}/uneven |
| luster/transparency | pearly, vitreous/translucent |
| color | clear, white, green, or sometimes gray |
| streak | white |
Properties in Thin Section
Apophyllite has negative relief, is colorless, and has very low birefringence. It has perfect (001) cleavage, and often exhibits anomalous interference colors. Uniaxial (+), ω = 1.535, ε = 1.537, δ = 0.002.
Crystallography
Apophyllite is tetragonal, a = 8.96, c = 15.78, Z = 2; space group $ \small{P \frac{4}{m} \frac{2_1}{n} \frac{2}{c}} $; point group $ \small{ \frac{4}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Apophyllite crystals typically have tetragonal prismatic faces with pinacoids and bipyramids. They often display pseudosymmetry, appearing to be combinations of cubes and octahedra.
Structure and Composition
Sheets in the apophyllite structure are composed of 4- and 8-member rings of SiO4 tetrahedra. Interlayer Ca, K, and F link the tetrahedral sheets.
Occurrence and Associations
Apophyllite is a secondary mineral that sometimes lines openings in basalt and other mafic igneous rocks. Associated minerals include zeolites, datolite, calcite, and pectolite.
1.3 Silicate Class: Chain Silicates
1.3.1 Pyroxenes
|
Pyroxene Group Minerals Orthopyroxene Series Clinopyroxene (Diopside) Series Pyroxene Solid Solutions Na- and Li-pyroxenes |
All pyroxenes have a structure based on chains of SiO4 tetrahedra linked by shared (bridging) oxygen. Octahedral cations (Ca,Na,Mg,Fe,Al) occupy sites between nonbridging oxygens of adjacent chains. Small amounts of Al sometimes replace tetrahedral Si.
Mineralogists divide pyroxenes into two groups based on their crystal symmetry. Orthopyroxenes (orthorhombic) have two principal end members: enstatite, Mg2Si2O6, and ferrosilite, Fe2Si2O6. Compositions between are generally called hypersthene but more specific names can be found in the literature.
Clinopyroxenes (monoclinic) are of several types, and include diopside–hedenbergite solid solutions, high-temperature polymorphs of hypersthene, and a number of other Ca- and Na-bearing species. The most important end member is diopside, CaMgSi2O6. Many natural clinopyroxenes are close to diopside composition, so the name diopside is sometimes (imprecisely) used to refer to any green or greenish black clinopyroxene. Petrologists use the names hypersthene, pigeonite, augite, omphacite, and aegirine to describe solid-solution clinopyroxenes having specific physical properties and compositions.
Except at very high temperature, a large solvus exists between monoclinic calcic pyroxenes and orthorhombic Ca-free pyroxenes. Consequently, many mafic rocks contain both clinopyroxene and orthopyroxene.
For more general information about the pyroxene group, see Section 6.4.6 in Chapter 6.
▪Enstatite Mg2Si2O6
Origin of Name
From the Greek word enstates, meaning “adversary,” which refers to this mineral’s resistance to melting.


Hand Specimen Identification
Occurrence in mafic rocks, vitreous luster, green or dark color, and two cleavages at about 90° to each other identify pyroxene, but distinguishing enstatite from other pyroxenes can be problematic unless it has its distinctive olive-green color and orthorhombic shape. It may also be confused with an amphibole, but amphiboles have two cleavages at about 60° to each other.
Figure 14.102 shows a single crystal of enstatite from Mogok, Myanmar, that displays its orthorhombic character well. The massive, undistinctive, bronzite in Figure 14.103 is, however, more typical. Bronzite is a variety of enstatite that contains Fe.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.2 to 3.5 |
| cleavage/fracture | near 90o cleavage angle, two perfect prismatic {210}/uneven |
| luster/transparency | pearly, vitreous/translucent |
| color | olive-green or gray |
| streak | white |
Properties in Thin Section
Enstatite is nearly colorless in thin section. If Fe has replaced some of the Mg, it may exhibit slight to pronounced pink to green pleochroism. An 86° cleavage angle, parallel extinction in prismatic section, and relatively low birefringence distinguish it from clinopyroxene. Biaxial (+), α = 1.657 , β = 1.659, γ = 1.665, δ = 0.008, 2V = 54°.
Crystallography
Enstatite is orthorhombic, a = 18.22, b = 8.81, c = 5.21, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2_1}{c} \frac{2_1}{a}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Enstatite is usually massive, but may be blocky, fibrous, or lamellar. Individual crystals may be prismatic or acicular.
Structure and Composition
Enstatite is one of two principal orthopyroxene end members; the other is ferrosilite, Fe2Si2O6. Enstatite and other pyroxenes contain chains of zigzagging SiO4 tetrahedra running parallel to the c-axis. Each tetrahedron shares two oxygens with neighbors in its chain and has one unshared oxygen at an apex pointing perpendicular to the c-axis. Pairs of chains face each other; Mg is located in four adjacent octahedral sites between unshared apices of tetrahedra.
Complete solid solution exists between enstatite and ferrosilite. Except at high temperature, only limited solid solution exists with calcic pyroxene. Mn, Cr, Al, and Ti may also be present in small amounts. See Section 6.4.6 in Chapter 6 for further discussion.
Occurrence and Associations
Enstatite is common in mafic igneous rocks, including gabbro, basalt, and norite, commonly associating with plagioclase and clinopyroxene. It is also found in some high-grade metamorphic rocks and is considered diagnostic for the granulite facies.
Varieties
Bronzite (Mg>>Fe) and hypersthene (Mg>Fe) are varietal names for Mg-Fe orthopyroxene.
Related Minerals
Enstatite is isostructural or isotypical with other pyroxenes. It is closely related to ferrosilite, Fe2Si2O6, and donpeacorite, (Mn,Mg)2Si2O6, two other pyroxenes. Most natural orthopyroxenes are predominantly enstatite–ferrosilite solid solutions with enstatite as their major component.
▪Diopside CaMgSi2O6
Origin of Name
From the Greek words dis and opsis, meaning “two” and “appearance,” referring to the fact that diopside appears different when viewed in different ways.


Hand Specimen Identification
An olive-green color and occurrence in marbles help identify diopside, although it may sometimes be confused with olivine. Diopside also occurs in association with other mafic minerals in mafic rocks, but in such occurrences, its color is more variable. Pyroxene cleavage – two cleavages at 86° to each other – aid identification.
Figure 14.104 shows green diopside crystals in a marble from the Adirondack Mountains. Figure 4.23 is a closeup view of diopside in Adirondack marble. The white mineral is calcite. Figure 13.35 is a photo of euhedral diopside, also from the Adirondacks. Figure 14.105 is a photo of green euhedral diopside with red garnet and darker-colored clinochlore.
Diopside may be confused with other pyroxenes or with hornblende, but the latter has two cleavages at 60° to each other. Diopside may contain substantial Fe, Al, or other impurities; exact composition cannot be determined without analytical data. Diopside is often white to green or pale green, especially in marbles; augite tends to be darker green or black.
Physical Properties
| hardness | 5.5 to 6.5 |
| specific gravity | 3.2 to 3.5 |
| cleavage/fracture | near 90o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | light green, variable |
| streak | white |
Properties in Thin Section
Diopside is colorless in thin section when Fe-free. With increasing iron content, it may become pleochroic in greens or browns. Higher birefringence and inclined extinction distinguish diopside and other clinopyroxenes from orthopyroxene. Grains are prismatic or blocky, depending on orientation. Extinction angle, optic sign, and 2V help distinguish the different clinopyroxenes, but telling them apart may be difficult. Biaxial (+), α = 1.665 , β = 1.672, γ = 1.695, δ = 0.030, 2V = 56° to 62°.
Crystallography
Diopside is monoclinic, a = 9.7, b = 8.9, c = 5.25, β = 105.83°, Z = 4; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Prismatic crystals often have a square or octahedral cross section. Diopside may also be massive or finely disseminated. Polysynthetic twins are common but are generally not be visible without a microscope.
Structure and Composition
The structure of diopside is similar to that of other pyroxenes. Chains of SiO4 tetrahedra run parallel to the c-axis, with octahedral Ca and Mg connecting opposing chains to each other. Ca and Mg occupy different structural sites; Ca:Mg ratios are always ≤1. Complete solid solution is possible between diopside (CaMgSi2O6), hedenbergite (CaFeSi2O6), and johannsenite (CaMnSi2O6). Small amounts of Al, Na, Ti, and Cr may be present.
Occurrence and Associations
Diopside is a common pyroxene. Diopside-rich clinopyroxene, generally called augite, is found in mafic and ultramafic igneous rocks, associated with plagioclase, hornblende, and olivine. Near end-member diopside is found in marbles where it is associated with calcite, quartz or forsterite, tremolite, scapolite, and garnet. It is also found in medium- and high-grade metamorphosed mafic rocks.

Varieties
Chrome diopside is a chromium-rich variety known for its vivid green color (Figure 14.106).
Related Minerals
All pyroxenes are closely related. Hedenbergite, CaFeSi2O6, is the iron end member of the diopside series. Augite, (Ca,Mg,Fe,Na)(Mg,Fe,Al)Si2O6, is a related pyroxene with slightly different structure. Pigeonite is a high-temperature, subcalcic pyroxene with compositions that approach those of Fe-Mg diopside.
▪Pigeonite (Ca,Mg,Fe)2Si2O6
Origin of Name
Named after the place where this mineral was originally found, Pigeon Cove, Minnesota.

Hand Specimen Identification
Occurrence as phenocrysts in a volcanic rock, form, two cleavages at 86°, and color may identify pigeonite, but this mineral occurs in other rocks, and distinguishing pigeonite from the other dark-colored pyroxenes is difficult. Only X-ray studies can make the distinction. Figure 14.107 shows a basalt from the moon that contains black crystals of pigeonite.
Physical Properties
| hardness | 6 |
| specific gravity | 3.4 |
| cleavage/fracture | near 90o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | pearly, vitreous/sometimes translucent |
| color | brown, green, black |
| streak | white |
Properties in Thin Section
Pigeonite is similar to other clinopyroxenes in thin section (see the optical properties of diopside) but has a lower 2V. Biaxial (+), a = 1.69 , β = 1.69, γ = 1.72, δ = 0.025, 2V = 0° to 32°.
Crystallography
Pigeonite is monoclinic, a = 9.73, b = 8.95, c = 5.26, β = 108.55°, Z = 4; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Rare euhedral crystals are prismatic. Pigeonite is most common as subhedral or anhedral grains in volcanic rock.
Structure and Composition
Pigeonite is similar in structure to other pyroxenes such as enstatite and diopside, and may contain the same impurities. It contains more Ca than enstatite and less than diopside, resulting in a slightly different structure.
Occurrence and Associations
Pigeonite is only found in high-temperature igneous rocks that have cooled rapidly. If it forms in plutonic rocks, it generally inverts to lower-temperature pyroxenes with cooling. Its former presence, however, may be inferred from textural features or from the presence of exsolved grains containing augite and orthopyroxene.
Related Minerals
Pigeonite is closely related to other pyroxenes, especially augite.
▪Augite (Ca,Mg,Fe,Na)(Mg,Fe,Al)(Si,Al)2O6
Origin of Name
From the Greek word augites, meaning “brightness,” referring to its shiny cleavage surfaces.


Hand Specimen Identification
Occurrence in mafic igneous rocks, form, two cleavages at 86°, and color identify pyroxene, but differentiating augite from other dark-colored pyroxenes is problematic without X-ray data. White to green or pale green pyroxenes are often diopside; augite tends to be dark green or black. Augite is occasionally confused with hornblende, but augite’s near 90o cleavage angle contrasts with hornblende’s 60o cleavage angle.
The two photos seen above show some classic examples of euhedral augite. Most augite, however, comprises small anhedral grains in plutonic or volcanic rocks. Figure 13.36 (from Chapter 13) shows an uncommon occurrence – a cluster of black augite crystals.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.2 to 3.4 |
| cleavage/fracture | near 90o cleavage angle, two perfect {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | black, dark green |
| streak | white |
Properties in Thin Section
Augite is similar to other clinopyroxenes in thin section. Grains are prismatic or blocky, depending on orientation. Color may be various shades of light brown, yellow-brown, or green. Augite may exhibit weak pleochroism. Extinction angle, optic sign, and 2V help distinguish the different clinopyroxenes, but telling them apart may be difficult. Biaxial (+), a = 1.671 to 1.735 , β = 1.672 to 1.741, γ = 1.703 to 1.761, δ = 0.030, 2V = 25° to 60°.
Crystallography
Augite is monoclinic, a = 9.8, b = 9.0, c = 5.25, β = 105°, Z = 4; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Individual augite crystals are typically poorly formed stubby black or green prisms with octagonal or square cross sections. Simple and polysynthetic twins may be present. Large euhedral crystals, like those seen in the two figures above, are relatively rare.
Structure and Composition
The structure of augite is similar to that of the other clinopyroxenes (see diopside structure). Its chemistry is, however, more complex and variable. Ca:Mg:Fe ratios vary. Significant solid solution occurs with jadeite (NaAlSi2O6;), aegirine (NaFeSi2O6), and Ca-Tschermak’s pyroxene (CaAl2SiO6). Ti, Li, Mn, and a number of other elements may also be present in small amounts.
Occurrence and Associations
Augite is the most common pyroxene found in mafic to intermediate igneous rocks, both plutonic and volcanic. Associated minerals include hornblende and plagioclase.

Related Minerals
Augite is equivalent in composition to diopside with many impurities. It is structurally and chemically closely related to other pyroxenes, especially pigeonite, and to pyroxenoids. Omphacite is a bright green variety of augite rich in Na and Al. Figure 14.110 show green omphacite in an eclogite from Norway. The rock also contains garnet, quartz, and a small amount of kyanite (near the center of the specimen).
▪Jadeite NaAlSi2O6
Origin of Name
Name of unknown origin. The term jade refers to either jadeite or to the amphibole, nephrite.
Hand Specimen Identification
Association with other high-pressure minerals, form, two (rarely seen) cleavages at near 90°, green color, and tenacity identify jadeite. It is distinguished from nephrite (a green amphibole) by its luster. An optical microscope may be needed to confirm identification.
The photos below show four examples of jadeite. Sometimes this mineral has a vivid green hue, but more subdued green colors, such as the color in Figure 14.113, are common. Jadeite has historically been used to make jewelry, in art projects, or as ornamental stone. Figure 14.114 shows examples of a figurine and cabochon made from jadeite.
Physical Properties
| hardness | 6.5 to 7 |
| specific gravity | 3.30 |
| cleavage/fracture | 86o cleavage angle, two perfect {110}/uneven |
| luster/transparency | vitreous, greasy/translucent |
| color | variable shades of green to white; may be emerald green |
| streak | white |
Properties in Thin Section
Jadeite is colorless to very pale green in thin section. Birefringence is low; anomalous blue interference colors are common, maximum interference colors are first-order red or yellow. Jadeite exhibits typical clinopyroxene shape and cleavage but has a higher 2V than most. Biaxial (+), α = 1.65 , β = 1.66, γ = 1.67, δ = 0.02, 2V = 70° to 75°.
Crystallography
Jadeite is monoclinic, a = 9.50, b = 8.61, c = 5.24, β = 110.46°, Z = 4; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Jadeite is usually granular, forming tenacious masses; less commonly, it is in prismatic or tabular crystals.
Structure and Composition
Jadeite is similar in structure to other clinopyroxenes and may contain some of the same impurities (see the composition of diopside). It forms limited solid solutions with aegirine, NaFeSi2O6, with omphacite, (Ca,Na)(Fe,Mg,Al)Si2O6, and with other pyroxene end members.
Occurrence and Associations
Jadeite is a high-pressure pyroxene found in metamorphic rocks of the blueschist facies. It is typically associated with other high-pressure minerals such as glaucophane, lawsonite, or aragonite, and with quartz and epidote. Omphacite, Na-rich augite that occurs in eclogites, is a solid-solution pyroxene that may be mostly jadeite.
Related Minerals
Jadeite is closely related to other Na-pyroxenes: aegirine, NaFeSi2O6; omphacite, (Ca,Na)(Fe,Mg,Al)Si2O6; and aegirine-augite, (Ca,Na)(Fe2+,Fe3+,Mg)Si2O6. It is chemically similar to nepheline, (Na,K)AlSiO4, and to albite, NaAlSi3O8.
▪Spodumene LiAlSi2O6
Origin of Name
From the Greek word spodoumenos, meaning “ashes.”


Hand Specimen Identification
Spodumene is typically found in pegmatites where it may form very large crystals such as the crystal seen in Figure 14.115. Prismatic cleavage, near 90o cleavage angle, hardness, light whitish color, and association help identify it, but it may be difficult to tell from feldspars or scapolite. This mineral commonly breaks into splintery cleavage fragments that have the appearance of a log or of petrified wood (Figure 14.116). Less commonly, spodumene occurs as clear gem crystals; Figures 14.117 and 14.118 below show two examples.
Physical Properties
| hardness | 6.5 to 7 |
| specific gravity | 3.15 |
| cleavage/fracture | near 90o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | typically light-colored white, gray, or colorless, less commonly, pink, green, yellow, purple, or tan |
| streak | white |
Properties in Thin Section
Spodumene is similar to other clinopyroxenes in thin section. Its occurrence in pegmatites and its small extinction angle (20° to 26°) help identify it. Biaxial (+), α = 1.65 , β = 1.66, γ = 1.67, δ = 0.02, 2V = 60° to 80°.
Crystallography
Spodumene is monoclinic, a = 9.52, b = 8.32, c = 5.25, β = 110.46°, Z = 4; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Prismatic crystals with well-developed {100} faces showing vertical striations are common for spodumene. Crystals are often polysynthetically twinned and may be very large. In some pegmatites they are tens of meters long. Spodumene also occurs as cleavable masses.
Structure and Composition
Spodumene is a pyroxene with structure similar to that of diopside. Minor Na substitutes for Li, Fe, Ca, Mn, Mg, and rare earths are also present in small amounts.
Occurrence and Associations
Spodumene is found in granitic pegmatites, where it associates with K-feldspar, muscovite, quartz, tourmaline, beryl, and lepidolite.


Varieties
Hiddenite is a name given to emerald-green spodumene; kunzite to lilac/pink spodumene; and triphane to colorless or yellow spodumene. The photos in these two figures show gemmy examples of triphane and kunzite from Afghanistan.
Related Minerals
Spodumene shares many properties with other pyroxenes. It is similar in composition to eucryptite, LiAlSiO4.
1.3.2 Amphiboles
|
Amphibole Group Minerals Monoclinic Amphiboles: Cummingtonite Series Monoclinic Amphiboles: Actinolite Series Hornblende Na-amphiboles Orthorhombic Amphiboles |
Amphiboles are double-chain silicates. They share many physical and chemical properties with pyroxenes. Major chemical variations mirror those of the pyroxene group.
Like the pyroxenes, amphiboles may be either monoclinic or orthorhombic. Calcic amphiboles are monoclinic; Ca-free amphiboles are generally orthorhombic. A solvus between calcic and noncalcic amphiboles results in many rocks containing two or, in some cases, three different amphibole species.
Many end-member amphiboles have specific names, the most important are listed in the box here (see hornblende entry, below, for additional names). The detailed descriptions that follow do not include gedrite and riebeckite because they are similar to the more common anthophyllite and glaucophane.
Petrologists use the name hornblende to refer to the common black amphibole found in many igneous and metamorphic rocks. Hornblendes have complex and highly variable chemistry; the formula in the box above only partially reflects the variations.
For more general information about the amphibole group, see Section 6.4.6 in Chapter 6.
▪Cummingtonite (Mg,Fe)7Si8O22(OH)2
Origin of Name
Named after Cummington, Massachusetts, its type locality.


Hand Specimen Identification
Prismatic bladed or acicular habit, two perfect cleavages intersecting at near 60° when viewed in basal section, color (if light brown or gray), and association identify cummingtonite. It may be confused with other amphiboles, especially anthophyllite and gedrite.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 2.9 to 3.2 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous, silky, fibrous/ transparent to translucent |
| color | light brown, gray, whitish, or green |
| streak | white |
Properties in Thin Section
Cummingtonite is colorless to pale green in thin section and exhibits weak pleochroism. Interference colors may be up to second order. Basal sections show typical amphibole cleavage displaying a 56° cleavage angle. Extinction is inclined, polysynthetic twinning is common, and birefringence is greater than for anthophyllite–gedrite. Biaxial (+), α = 1.644 , β = 1.657, γ = 1.674, δ = 0.030, 2V = 80° to 90°.
Crystallography
Cummingtonite is monoclinic, a = 9.51, b = 18.19, c = 5.33, β = 101.83°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Cummingtonite forms prismatic, fibrous crystals; aggregates of radiating fibers or blades are common. See Figures 14.119 and 14.120, above.
Structure and Composition
Cummingtonite, like other amphiboles, has a double-chain structure. SiO4 tetrahedra are linked to make double chains that run parallel to the c-axis. Each tetrahedron shares two or three oxygen with neighbors, and has an unshared oxygen at the vertex pointing perpendicular to c. Chains are paired; unshared oxygens point toward each other and are bonded to the five octahedral cations occupying sites between them. A complete solid solution series exists between Mg-cummingtonite, Mg7Si8O22(OH)2, and grunerite, Fe7Si8O22(OH)2. The name cummingtonite is given to intermediate compositions, (Mg,Fe)7Si8O22(OH)2, with Mg > Fe. Substantial Mn may replace Mg; Al and Ca may be present in small amounts.
Occurrence and Associations
Cummingtonite occurs in mafic or marly medium-grade metamorphic rocks. Common associated minerals include other amphiboles (hornblende, actinolite, or anthophyllite), garnet, plagioclase, and cordierite. Cummingtonite also occurs in a few rare kinds of igneous rocks.

Varieties
Amosite is an asbestiform amphibole similar to Fe-rich cummingtonite. Figure 14.121 shows an example.
Related Minerals
Cummingtonite is closely related to the other amphiboles and is polymorphic with members of the anthophyllite series.
▪Grunerite Fe7Si8O22(OH)2
Origin of Name
Named after L. E. Grüner, a nineteenth-century mineralogist who first analyzed grunerite.


Hand Specimen Identification
Green color, acicular or bladed crystals, radiating habit, and association with other Fe-rich minerals help identify grunerite. It can, however, be difficult to distinguish from other amphiboles, especially cummingtonite. If crystals are large enough, they show two prominent cleavages at 56o to each other, confirming amphibole identity but not the species.
Commonly, grunerite crystals are in the form of fine needles or blades, as in the samples of Figures 14.122 adn 14.123. Needles may be aligned with either parallel or radiating textures, but radiating is more diagnostic.
Physical Properties
| hardness | 6 |
| specific gravity | 3.1 to 3.6 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | dark green or brown |
| streak | white |
Properties in Thin Section
Grunerite is similar to other members of the cummingtonite–grunerite series (see cummingtonite optics), but exhibits less pleochroism than cummingtonite, has an extinction angle of 10° to 15° prismatic cleavage, and may show interference colors up to third order. Biaxial (-), α = 1.69 , β = 1.71, γ = 1.73, δ = 0.040, 2V = 80° to 90°.
Crystallography
Grunerite is monoclinic, a = 9.6, b = 18.3, c = 5.3, β = 101.8°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Grunerite typically forms fibrous, bladed, or columnar crystals, often radiating.
Structure and Composition
Grunerite is an end member of the cummingtonite–grunerite series. Structure and composition are analogous to cummingtonite. The name grunerite is, by definition, restricted to compositions close to end-member Fe7Si8O22(OH)2.
Occurrence and Associations
Grunerite is found with Fe-rich minerals such as magnetite, hematite, minnesotaite, hedenbergite, fayalite, or garnet in metamorphosed Fe-rich sediments.
Related Minerals
Grunerite is closely related to the other amphiboles, especially cummingtonite.
▪Tremolite Ca2Mg5Si8O22(OH)2
Origin of Name
Named after Val Tremola, Switzerland, where it was first found.



Hand Specimen Identification
Occurrence in metacarbonates, perfect prismatic cleavages, 56° cleavage angle when viewed in basal section, fibrous/bladed or thin columnar crystals, and generally very light color identify tremolite. Cleavage angle distinguishes it from pyroxenes and pyroxenoids; light color distinguishes it from hornblende. It may sometimes be confused with vesuvianite or wollastonite.
The fragment of tremolite in Figure 14.124 shows amphibole cleavage well; otherwise the specimen is quite nondescript. This is typical for tremolite. When euhedral, tremolite commonly forms white blades, sometimes in masses as seen in the photo in Figure 14.125. Figure 14.126 shows an uncommon occurrence – a transparent single needle of tremolite.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.0 to 3.3 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | typically white, less commonly light green |
| streak | white |
Properties in Thin Section
Generally colorless when Fe-free, tremolite may be green and pleochroic when Fe is present. Amphibole cleavage angles (56° and 124°), 10° to 21° extinction angle in prismatic section, large 2V, and upper first- to second-order interference colors identify tremolite. Biaxial (-), α = 1.608 , β = 1.618, γ = 1.630, δ = 0.022, 2V = 85°.
Crystallography
Tremolite is monoclinic, a = 9.86, b = 18.11, c = 5.34, β = 105.00°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Tremolite is typically prismatic. It may be in radiating or parallel blades, fibrous, asbestiform, or columnar. It is commonly twinned parallel to {100}.
Structure and Composition
Tremolite, Ca2Mg5Si8O22(OH)2, is the Mg end member of the calcic amphibole series. Complete solid solution exists between tremolite and Fe-actinolite, Ca2Fe5Si8O22(OH)2. Intermediate compositions are simply termed actinolite. Like other amphiboles, tremolite has a double-chain structure. SiO4 tetrahedra are linked to make double chains that run parallel to the c-axis. Each tetrahedron shares two or three oxygen with neighbors and has an unshared oxygen at the vertex pointing perpendicular to c. Chains are paired; unshared oxygens point toward each other and are bonded to the five octahedral cations occupying sites between them. The two larger octahedral sites are occupied by Ca. Other alkalis and alkaline earths may substitute in small amounts for Ca, and some Al may be present in either the octahedral or tetrahedral sites. If impurities are present in sufficient quantities, the amphibole becomes dark and, in the absence of analytical data, we call it hornblende.
Occurrence and Associations
Tremolite is one of the first minerals to form when impure carbonates are metamorphosed. It is associated with calcite, dolomite, talc, quartz or forsterite, diopside, and phlogopite.

Varieties
Hexagonite is a red to pink, lilac or purple Mn-rich variety of tremolite (Figure 14.127).
Related Minerals
All amphiboles are structurally similar. Tremolite is closely related to Fe-actinolite Ca2Fe5Si8O22(OH)2, the other principal calcic amphibole end member.
▪Actinolite Ca2(Fe,Mg)5Si8O22(OH)2
Origin of Name
From the Greek word actis (ray), referring to its common habit of radiating needles.


Hand Specimen Identification
A bladed, columnar, or needle-like habit, forming in masses, prismatic cleavages, 56° and 124° cleavage angles, and distinctive green color usually serve to identify actinolite. It is sometimes confused with epidote because of its green color. Mg:Fe ratios of actinolite may vary; exact composition cannot be determined in hand specimen.
The photo in Figure 14.128 shows a mass of classic bladed green actinolite. Most actinolite specimens have an appearance quite similar to this one. The photo in Figure 14.129 shows a rare occurrence – a spectacular cluster of parallel euhedral actinolite crystals from Namibia.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.0 to 3.3 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | dark green |
| streak | white |
Properties in Thin Section
Actinolite is similar to tremolite in thin section (see tremolite), but is generally more strongly colored and pleochroic. Biaxial (-), α = 1.66 to 1.67, β = 1.62 to 1.68, γ = 1.63 to 1.69, δ = 0.03, 2V = 70° to 80°.
Crystallography
Actinolite is monoclinic, a = 9.84, b = 18.05, c = 5.27, β = 104.7°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Actinolite typically forms needles or blades – either radiating or in parallel aggregates – or columnar masses.
Structure and Composition
Actinolite is the name given to green amphiboles with compositions intermediate between tremolite, Ca2Mg5Si8O22(OH)2, and Fe-actinolite, Ca2Fe5Si8O22(OH)2. Mn, Al, F, and Cr are sometimes present in minor amounts. Actinolite has the same structure as tremolite and other calcic amphiboles.
Occurrence and Associations
Actinolite is characteristic of medium-grade metamorphosed mafic rocks. It is one of the minerals that give greenschists their characteristic color. Associated minerals typically include albite, epidote, chlorite, and quartz.


Varieties
Byssolite is the name given to fibrous actinolite; Figure 14.130 shows an example. Nephrite, a mineral that may be called jade if gemmy, is an Na-Al variety of actinolite. Figure 14.131 show several different artifacts made from nephrite.
Related Minerals
All amphiboles are structurally similar. Actinolite is closely related to tremolite, Ca2Mg5Si8O22(OH)2, the other calcic amphibole end member.
▪Hornblende (K,Na)0-1(Ca,Na,Fe,Mg)2(Mg,Fe,Al)5(Si,Al)8O22(OH)2
Origin of Name
From the German word horn (horn) and blenden (blind), referring to its luster (like the horn of an animal) and its lack of value (blenden means deceiving).


Hand Specimen Identification
The two photos show typical specimens of hornblende, the most common amphibole. It is generally vitreous, black, has typical amphibole habit, and near 60o cleavage angle. It is occasionally confused with augite, black pyroxene, but augite has a near 90o cleavage angle. In the absence of compositional information, we use the name hornblende for any black amphibole.
The euhedral crystals seen in Figure 14.132 are attractive but not typical for hornblende. More commonly it occurs in anhedral crystals like those seen in Figure 14.133.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.0 to 3.5 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/translucent |
| color | black or less commonly dark green |
| streak | white |
Properties in Thin Section
Hornblende may be various shades of brown, green, blue-green, or yellow-brown in thin section. Moderate to strong pleochroism is typical. Cross sections may be pseudohexagonal or diamond shaped. It may appear superficially like biotite, but has two good cleavages, and generally higher birefringence. 56° and 124° cleavage angles distinguish it from pyroxenes. Biaxial (-), α = 1.65 , β = 1.66, γ = 1.67, δ = 0.02, 2V = 50° to 80°.
Crystallography
Hornblende is monoclinic, a = 8.97, b = 18.01, c = 5.33, β = 105.75°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Hornblende may be massive or prismatic and is sometimes bladed, columnar, or fibrous. Euhedral crystals are often prismatic with a pseudohexagonal cross section. {100} contact twins are common.
Structure and Composition
Hornblende structure is similar to other amphiboles, except that a large site between tetrahedral chains, vacant in most of them, is partly occupied by Na+ or K+. Thus, hornblende contains seven octahedral cations, and additional Na+ and/or K+ in 10-12 fold coordination.
Hornblende composition varies greatly. Many end members have names; some of the more commonly used ones are:
edenite Ca2NaMg5(AlSi7)O22(OH)2
ferro-edenite Ca2NaFe5(AlSi7)O22(OH)2
pargasite Ca2NaMg4Al(Al2Si6)O22(OH)2
ferro-pargasite Ca2NaFe4Al(Al2Si6)O22(OH)2
tschermakite Ca2Mg3Al2(Al2Si6)O22(OH)2
ferrro-tschermakite Ca2Fe3Al2(Al2Si6)O22(OH)2
tremolite Ca2(Mg,Fe)2Si8O22(OH)2
ferro-actinolite Ca2(Fe,Mg5)Si8O22(OH)2
glaucophane Na2Mg3Al2Si8O22(OH)2
kaersutite Ca2Na(Mg,Fe)4Ti(Al2Si6)O22(OH)2
Besides compositional variations described by the end members listed above, some hornblende varieties include F– or O2- substituting for (OH)–, or Fe3+ substituting for Fe2+
Occurrence and Associations
Hornblende is found in many kinds of igneous rocks covering a wide range of composition. It is usually associated with plagioclase and may coexist with quartz or with mafic minerals such as pyroxene or olivine. It is especially common in igneous rocks of intermediate composition such as syenite or diorite. Hornblende is also found in metamorphosed mafic rocks, especially in amphibolites (that typically contain hornblende and plagioclase as dominant minerals).
Related Minerals
All of the amphiboles are closely related in composition and structure. Hornblende has a more variable composition than most of the others.
▪Glaucophane Na2Mg3Al2Si8O22(OH)2
Origin of Name
From Greek words meaning “to appear bluish.”


Hand Specimen Identification
Association with other high-pressure minerals in blueschists, often fibrous habit, near 60° cleavage angle, and blue color are distinctive of glaucophane and related Na-amphiboles crossite and riebeckite.
Figure 14.134 shows a blueschist that is mostly made of blue glaucophane. If you enlarge the view, you can see that some small grains of green pyroxene (omphacite) are present, too. This is a typical glaucophane occurrence; this amphibole rarely forms coarse crystals. Figure 14.135 shows the same two minerals, glaucophane and omphacite, but the glaucophane forms slender blue needles. Figure 8.82 is a photo of glaucophane with fuchsite (Cr-rich mica) from France.
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 3.1 to 3.2 |
| cleavage/fracture | near 60° cleavage angle, two perfect prismatic {110}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | blue or sometimes light gray |
| streak | white to very light blue |
Properties in Thin Section
Glaucophane may be difficult to tell from other blue amphiboles (such as riebeckite or crocidolite). It is colorless to blue or violet in thin section and often strongly pleochroic. Interference colors may range up to low second order, but are sometimes masked by mineral color. It exhibits typical amphibole cleavage and often forms fine prisms or needles with diamond-shaped cross sections. Biaxial (-), α = 1.66 , β = 1.67, γ = 1.65, δ = 0.01 to 0.02, 2V = 0° – 50°.
Crystallography
Glaucophane is monoclinic, a = 9.78, b = 17.80, c = 5.30, β = 103.76°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Acicular, asbestiform, columnar, or fibrous habit characterizes glaucophane.
Structure and Composition
Glaucophane has a structure similar to the calcic amphiboles (see tremolite structure). Although glaucophane has end-member composition Na2Mg3Al2Si8O22(OH)2, most natural samples contain substantial Fe and Al. Fe2+ replaces Mg2+ and Fe3+ replaces Al3+. If Fe2+ and Fe3+ replace most of the Mg2+ and Al3+, the amphibole becomes riebeckite. In riebeckite, some Na+ enters a normally vacant interlayer site between tetrahedral chains. Compositions intermediate between glaucophane and riebeckite are called crossite.
Occurrence and Associations
Glaucophane is a high-pressure metamorphic mineral characteristic of the blueschist facies. Other blueschist minerals commonly found with glaucophane include jadeite, lawsonite, and aragonite.
Related Minerals
Glaucophane is similar in structure and chemistry to all amphiboles, but in particular the sodic amphiboles: riebeckite, Na2Fe3Fe2Si8O22(OH)2; eckermannite, NaNa2Mg4AlSi8O22(OH)2; and arfvedsonite, NaNa2Fe5Si8O22(OH)2. In the latter two, substantial Na+ occupies a normally unoccupied interlayer site between tetrahedral chains.
▪Anthophyllite (Mg,Fe)7Si8O22(OH)2
Origin of Name
From the Latin word anthophyllum, meaning “clove leaf,” referring to this mineral’s color.


Hand Specimen Identification
Anthophyllite is characterized by its whitish to clove-brown color, usual prismatic or acicular habit, and prismatic cleavages with a 54° to 55° cleavage angle. It is, however, difficult to distinguish from other light-colored amphiboles such as gedrite, grunerite, or cummingtonite. Some samples of anthophyllite are fibrous to asbestiform.
Figure 14.136 shows a sample from Finland that contains stellate (star-like) anthophyllite needles that radiate from points. Figure 14.137 shows a different specimen from Finland that contains sprays of anthophyllite needles that are more subparallel. In this second photo, green actinolite accompanies the light colored anthophyllite. Figure 3.65, from Chapter 3, is a photo closeup of anthophyllite needles.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 2.9 to 3.2 |
| cleavage/fracture | near 60o cleavage angle, two perfect prismatic {210}, poor (100)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | brown to green |
| streak | white |
Properties in Thin Section
In thin section, anthophyllite is colorless to pale brown or green and may be weakly pleochroic. It shows typical amphibole cleavage angles (56° and 124°) and up to second-order interference colors. It is difficult to tell from gedrite, but parallel extinction distinguishes it from clinoamphiboles. Biaxial (+ or -), α = 1.60, α = 1.62, γ = 1.63, δ = 0.03, 2V = 65° to 90°.
Crystallography
Anthophyllite is orthorhombic, a = 18.56, b = 18.01, c = 5.28, Z = 4; space group $ \small{P \frac{2}{n} \frac{2}{m} \frac{2}{a}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Anthophyllite crystals are prismatic, fibrous, bladed, or columnar with diamond-shaped cross sections.
Structure and Composition
Anthophyllite is part of a solid-solution series extending from Mg7Si8O22(OH)2 toward Fe7Si8O22(OH)2. Although compositionally identical to the monoclinic cummingtonite–grunerite amphiboles, anthophyllite is orthorhombic. Anthophyllite is usually Mg-rich. Al and Na may be present in anthophyllite; if Al content is great enough, the amphibole is called gedrite. The structure of anthophyllite is similar to that of other amphiboles.
Occurrence and Associations
Anthophyllite is found in low-grade Mg-rich metamorphic rocks where it may be associated with cordierite. It is sometimes secondary after high-temperature minerals such as pyroxene and olivine, and is common in some serpentinites.
Varieties
Amosite is the name given to asbestiform anthophyllite.
Related Minerals
Anthophyllite is similar to all the other amphiboles, especially gedrite, (Mg,Fe,Mn)2(Mg,Fe,Al)5(Si,Al)8O22(OH)2, and holmquistite, Li2(Mg,Fe)3Al2Si8O22(OH)2. It is polymorphic with cummingtonite.
1.3.3 Pyroxenoids
| Pyroxenoid Group Minerals wollastonite CaSiO3 rhodonite MnSiO3 pectolite NaCa2(SiO3)3H |
Pyroxenoids are chain silicates having structures similar, but not identical, to pyroxenes. In pyroxene chains, SiO4 tetrahedra zigzag back and forth. In common pyroxenoids, every other tetrahedron has the same orientation; the chains have a repeat distance of two tetrahedra, approximately 5.2 Å. In pyroxenoids, repeat distances involve three or more tetrahedra, sometimes in complex arrangements. Ca2+, Mn2+, and other cations, bonded to unshared chain oxygens, occupy distorted octahedral sites between chains. The less regular structures of pyroxenoids have less symmetry than pyroxenes; pyroxenoids are triclinic, whereas pyroxenes are monoclinic or orthorhombic.
▪Wollastonite CaSiO3
Origin of Name
Named after W. H. Wollaston (1766–1828), who discovered palladium and rhodium, invented the reflecting goniometer, and developed the camera lucida.



Hand Specimen Identification
Wollastonite’s restricted occurrence in high-temperature metacarbonates, white color, and common splintery or bladed habit due to two perfect cleavages make it distinctive. Tremolite has many features in common with wollastonite, but wollastonite can be distinguished by its two perfect cleavages about 84° apart (c.f., 56° in tremolite). Pectolite may be confused with wollastonite; both are typically white with similar lusters and habits.
Figure 14.138 shows a large piece of wollastonite containing subparallel blades. In Figure 14.139, wollastonite blades/needles form sprays. Figure 14.140 is a classic sample of marble from the Adirondack Mountains, New York, that contains stubby blades/tabs of white wollastonite, equant grains of green diopside, and minor red garnet.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 3.1 |
| cleavage/fracture | near 90o cleavage angle, perfect (100) and (001), good (102)/uneven |
| luster/transparency | silky, vitreous/translucent |
| color | white |
| streak | white |
Properties in Thin Section
In thin section, wollastonite is clear and has low birefringence. It has two good-perfect cleavages in cross section and one in prismatic section, and it has near-parallel extinction. Wollastonite is distinguished from tremolite, pectolite, and diopside by its lower birefringence. Biaxial (-), α = 1.620 , β = 1.632, γ = 1.634, δ = 0.014, 2V = 39°.
Crystallography
Wollastonite is triclinic, a = 7.94, b = 7.32, c = 7.07, α = 90.03°, β = 95.37°, γ = 103.43°, Z = 4; space group $ \small{P\overline{1}} $; point group $ \small{\overline{1}}$.
Habit
Wollastonite typically forms cleavable masses or fibrous aggregates. Tabular or prismatic crystals are also common.
Structure and Composition
Wollastonite, generally close to 100% CaSiO3, may contain minor Mn substituting for Ca. Its structure is similar to other pyroxenoids and to pyroxenes (see the introduction to pyroxenoid group minerals and enstatite).
Occurrence and Associations
Wollastonite is common in high-grade marbles and other calcareous metamorphic rocks, especially contact metamorphic rocks. Common associated minerals are calcite, dolomite, tremolite, epidote, garnet, diopside, and vesuvianite.
Related Minerals
Wollastonite and other pyroxenoids are related to pyroxenes in both chemistry and structure. Other pyroxenoids include rhodonite, MnSiO3; pectolite, NaCa2(SiO3)3H; and bustamite, (Mn,Ca,Fe)SiO3. Pseudowollastonite, a high-temperature polymorph, is similar to wollastonite, and several other wollastonite polymorphs are known.
▪Rhodonite MnSiO3
Origin of Name
From the Greek word rhodon, meaning “rose,” in reference to rhodonite’s color.


Hand Specimen Identification
Rhodonite is one of the few pink minerals and has a nearly perfect 90° cleavage angle. Occurence with other Mn-minerals aids identification. It is occasionally confused with rhodochrosite but is harder and has a different habit.
Figure 14.141 shows typical anhedral rhodonite. Its pink color and hardness are keys to identification. Figure 14.142 shows a spectacular specimen of euhedral rhodonite and quartz. The color of the rhodonite in this specimen is uncommon but there are few other minerals that can have this hue. Figure 10.65 is a photo of another rhodonite crystal with the same vivid color.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 3.5 to 3.7 |
| cleavage/fracture | near 90o cleavage angle, perfect {110}, perfect {110}/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | pink, occasionally red; weathers to dark-colored Mn-oxide |
| streak | white |
Properties in Thin Section
Rhodonite is weakly pleochroic, colorless to light pink in thin section; maximum interference color is first-order yellow. Inclined extinction, high index of refraction, and low birefringence help identify it. Biaxial (+), α = 1.717 , β = 1.720, γ = 1.730, δ = 0.013, 2V = 63° to 76°.
Crystallography
Rhodonite is triclinic, a = 7.68, b = 11.82, c = 6.71, α = 92.35°, β = 93.95°, γ = 105.67°, Z = 2; space group $ \small{P\overline{1}} $; point group $ \small{\overline{1}}$.
Habit
Cleavable masses or discrete grains are typical of rhodonite; thick tabular crystals, such as those seen in Figure 14.142, above, are uncommon but are diagnostic.
Structure and Composition
The rhodonite structure is similar to that of other pyroxenoids (see the introduction to the pyroxenoid group minerals). Rhodonite always contains some Ca substituting for Mn. If Ca content is great enough, it becomes bustamite. Fe and Zn may be present as well.
Occurrence and Associations
Rhodonite is found in manganese deposits and some iron formations. It is often found with Zn-minerals. Other associated minerals include the Mn-minerals rhodochrosite, bustamite, pyrolusite, tephroite, zincite, willemite, and also calcite and quartz.
Related Minerals
Rhodonite is similar in composition and structure to other pyroxenoids, especially pyroxmangite, (Mn,Fe)SiO3, and bustamite, (Mn,Ca,Fe)SiO3. Pyroxmangite, however, contains less Ca and more Fe, and bustamite contains significantly more Ca.
▪Pectolite NaCa2(SiO3)3H
Origin of Name
From the Greek word pectos, meaning “well put together.”


Hand Specimen Identification
Light color, occurrence in fibrous radiating masses, and common silky or translucent luster help identify pectolite. It has two cleavages at near 90o, and breaks into sharp acicular fragments when cleaved. Pectolite is occasionally confused with wollastonite or zeolites.
The two photos seen here are samples of pectolite from New Jersey. In Figure 14.143, the crystals are in clearly seen radiating sprays. In Figure 14.144, they are in radiating sprays too, but the radiating crystals have formed globular balls and have a botryoidal appearance.
Physical Properties
| hardness | 5 |
| specific gravity | 2.9 |
| cleavage/fracture | near 90o cleavage angle, two perfect prismatic {100}, perfect {001} |
| luster/transparency | silky/translucent |
| color | white |
| streak | white |
Properties in Thin Section
Pectolite is colorless in thin section, has moderate birefringence and relief, and shows two perfect cleavages with parallel extinction. Biaxial (+), α = 1.59 , β = 1.61, γ = 1.63, δ = 0.04, 2V = 35° to 63°.
Crystallography
Pectolite is triclinic, a = 7.99, b = 7.04, c = 7.02, α = 90.05°, β = 95.28°, γ = 102.47°, Z = 2; space group $ \small{P\overline{1}} $; point group $ \small{\overline{1}}$.
Habit
Pectolite is typically fibrous or acicular. Acicular radiating crystals forming compact masses, such as those seen in the two photos above, are common.
Structure and Composition
Pectolite is similar in structure to wollastonite. Twisted chains of SiO4 tetrahedra run parallel to the b-axis and are connected by Na and Ca in octahedral coordination. Pectolite may contain small amounts of Fe, K, or Al.
Occurrence and Associations
Pectolite is usually a secondary mineral, resembling zeolites in appearance and occurrence. It is typically found as crusts, in cavities, or along joints in basalt. Associated minerals include zeolites, calcite, and prehnite. It is occasionally found as a primary mineral in alkalic igneous rocks or in calcic metamorphic rocks.

Varieties
Larimar (Figure 14.145) is a gemmy blue variety of pectolite found only in the Dominican Republic.
Related Minerals
Pectolite is structurally and chemically related to other pyroxenoids and pyroxenes.
1.4 Silicate Class: Ring Silicates
|
Tourmaline |
Ring silicates have structures in which all silicon tetrahedra are in hexagonal (6-membered) rings. Using this definition, tourmaline is the only common example. The very rare minerals dioptase, CuSiO2(OH)2, and benitoite, BaTiSi3O9, are also ring silicates. Mineralogists sometimes group beryl and cordierite with tourmaline because the two minerals, like tourmaline, contain hexagonal rings of tetrahedra. But, in beryl and cordierite, tetrahedral rings are connected by additional silica tetrahedra that are not in rings.
For more general information about the ring silicates, see Section 6.4.6 in Chapter 6.
▪Tourmaline (Na,Ca)(Fe,Mg,Al,Li)3Al6(BO3)3Si6O18(OH)4
Origin of Name
From the Sinhalese word toramalli, meaning “brown,” the color of some tourmaline gemstones from Ceylon.
Hand Specimen Identification
Common occurrence in pegmatites, prismatic crystal habit, pseudohexagonal cross section, vitreous luster, hardness, and conchoidal fracture are characteristic and typically identify tourmaline. But, tourmaline comes in many different colors. Black tourmaline may be confused with hornblende; small crystals may superficially resemble staurolite.
Figure 14.146 is a photo of common black tourmaline, a variety called schorl, in a pegmatite. The crystal in the center of the sample gives a hint of tourmaline‘s pseudohexagonal symmetry. Figure 14.147 also shows schorl. The pseudosymmetry is present in this specimen too, but is hard to see because of the angle of view. Figure 14.148 is a photo of rubellite, a red variety of tourmaline; and Figure 14.149 is a photo of elbaite, a green variety.
Physical Properties
| hardness | 7 to 7.5 |
| specific gravity | 2.9 |
| cleavage/fracture | poor {101}, poor {110}/ subconchoidal |
| luster/transparency | vitreous, sometimes resinous/translucent |
| color | variable black, blue, green, red, colorless; often zoned |
| streak | white |
Properties in Thin Section
In thin section, tourmaline’s color and pleochroism are strong and variable: black, brown, green, blue, yellow, red, or pink. The color usually masks the birefringence. Pseudohexagonal or triangular cross sections are typical. Tourmaline is uniaxial (-), typically ω = 1.645 to 1.670, ε = 1.625 to 1.640, δ = 0.020 to 0.030.
Crystallography
Tourmaline forms trigonal crystals. Its unit cell dimensions are a = 15.84 to 1.603, c = 7.10 to 0.722, Z = 3; space group $ \small{R3m}$; point group $ \small{3m}$.
Habit
Elongate trigonal prisms, sometimes appearing hexagonal, with vertical striations are common. Cross sections appear hexagonal or ditrigonal, with or without some rounded angles between faces. Tourmaline may occur as parallel or radiating crystal aggregates or may be massive and compact.
Structure and Composition
One of the few common boron minerals, The basic structure consists of rings containing six (SiO4) tetrahedra and octahedra containing Al, Mg, or Fe. The rings are connected to borate groups (BO3) and to O or OH at the corners of the octahedra. Cations such as Na+ occupy positions in the center of the rings.
Occurrence and Associations
Tourmaline is a common accessory mineral in many granitic igneous rocks and in some metamorphic rocks. Rarely, it is found as detrital grains in sediments. It commonly associates with quartz and K-feldspar. It may be a major mineral in pegmatites, where it is often associated with lepidolite, beryl, apatite, spodumene, or fluorite.


Varieties
Tourmaline’s composition is highly variable, leading to many different colored varieties. Fe-rich varieties are black; Mg-rich varieties are often brown or yellow; Li-rich varieties may be blue or green; Mn-rich varieties are pink or red. These varieties are named according to their colors:
xx•black-blue tourmalines are schorl
xx•brown-yellow tourmalines are dravite
xx•variable blue tourmalines are elbaite
xx•red or pink tourmalines are rubellite
Figure 14.150 shows some of the different colored tourmalines that have been shaped and polished to make cabochon gems. Figure 14.151 shows examples of multicolored watermelon tourmaline.
Related Minerals
Tourmaline is structurally related to beryl, Be3Al2Si6O18; cordierite, (Mg,Fe)2Al4Si5O18; dioptase, CuSiO2; and benitoite, BaTiSi3O9. All contain hexagonal rings of SiO4 tetrahedra.
1.5 Silicate Class: Isolated Tetrahedral Silicates
1.5.1 Garnets
|
Garnet Group Minerals Pyralspite Series Ugrandite Series |
Garnet composition is quite variable but all garnets have chemical formula A3B2Si3O12. Their structures consist of isolated (SiO4)4- tetrahedra linked to distorted octahedrons (B atoms; most commonly Al3+ or Fe3+) and to distorted dodecahedrons (A atoms; most commonly Ca2+, Mg2+, Fe2+, or Mn2+). Mineralogists conveniently divide garnets into two series, the pyralspites (pyrope–almandine-spessartine) and the ugrandites (uvarovite-grossular–andradite). Complete solid solution exists within each series, but only limited solid solution occurs between the two.
Garnets are nearly exclusively found in metamorphic rocks. Chapter 8 discusses many different aspects of garnet occurrences.
▪Pyrope Mg3Al2Si3O12
Origin of Name
From the Greek word pyropos, meaning “fiery,” a reference to this mineral’s luster.


Hand Specimen Identification
Hardness, lack of cleavage, vitreous luster, and crystal shape (when euhedral) identify garnets. Some varieties have distinct colors, and different species can sometimes be identified by color or association. Pyrope (Mg-garnet) is most often red. It can be mistaken for almandine, the most common kind of red garnet. Garnets are complex solid-solution minerals, and determining exact composition requires analytical data.
Physical Properties
| hardness | 7 |
| specific gravity | 3.54 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/often translucent to transparent |
| color | red and occasionally other colors including black |
| streak | white |
Properties in Thin Section
Pyrope is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. It is isotropic, n = 1.71.
Crystallography
Pyrope is cubic, a = 11.46, Z = 8; space group $ \small {I \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Equant grains, sometimes displaying dodecahedral or trapezohedral faces, characterize pyrope and other garnets. Massive occurrences are rare.
Structure and Composition
The name pyrope refers to garnets close in composition to the Mg end member of the pyralspite series. The structure of pyrope is similar to the structures of other garnets.
Occurrence and Associations
Pyrope is only stable in high-pressure rocks and is found in eclogites and other mafic and ultramafic rocks from deep within Earth. Commonly associated minerals include olivine, pyroxene, spinel, and occasionally diamond.
▪Almandine Fe3Al2Si3O12
Origin of Name
From Alabanda, a Middle Eastern trade center where garnets were cut and polished in the first century A.D.



Hand Specimen Identification
Hardness, lack of cleavage, luster, and crystal shape (when euhedral) identify garnets. Garnets are, however, solid-solution minerals, and determining exact composition requires analytical data. Some varieties have distinct colors, and different species can sometimes be distinguished by color or association. Almandine typically has a distinctive deep wine-red color that can be seen in the three photos above. In Figure 14.156, the almandine is accompanied by green diopside and dark purplish clinochlore. This pretty specimen comes from the Aosta Valley, Italy.
Physical Properties
| hardness | 7 |
| specific gravity | 4.33 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/often translucent to transparent |
| color | deep red |
| streak | white |
Properties in Thin Section
Almandine is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. It is isotropic, n = 1.83.
Crystallography
Almandine, like all garnets, is cubic, a = 11.46, Z = 8; space group $ \small {I \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Almandine is characterized by euhedral to subhedral equant grains displaying dodecahedral or trapezohedral faces. Massive occurrences are rare.
Composition and Structure
Almandine, the Fe end member of the garnet pyralspite series, may contain appreciable amounts of Ca, Mg, or Mn replacing Fe. It has the same structure as other garnets.
Occurrence and Associations
Almandine is a common mineral in medium- and high-grade metamorphic rocks. It is often found with quartz, feldspars, micas, staurolite, cordierite, chloritoid, tourmaline, and kyanite or sillimanite.
▪Spessartine Mn3Al2Si3O12
Origin of Name
From Spessart, a district in Germany.

Hand Specimen Identification
Hardness, lack of cleavage, luster, and crystal shape (when euhedral) identify garnets. Some varieties have distinct colors, but spessartine does not. It usually can only be identified by chemical analysis, although association with other Mn minerals is suggestive. Figure 14.157 shows a mass of euhedral spessartine crystals from Fujian Province, China.
Physical Properties
| hardness | 7 |
| specific gravity | 4.19 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/ commonly translucent to transparent |
| color | red, reddish orange, yellowish brown, reddish brown, or brown |
| streak | white |
Properties in Thin Section
Spessartine is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. Isotropic, n = 1.80.
Crystallography
Spessartine, like all garnets, is cubic, a = 11.62, Z = 8; space group $ \small {I \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Euhedral to subhedral equant grains displaying dodecahedral or trapezohedral faces characterize spessartine. Massive occurrences are rare.
Structure and Composition
Spessartine is the Mn end member of the pyralspite series. Natural spessartine may contain appreciable amounts of Ca, Mg, or Fe replacing Mn. Spessartine has the same structure as other garnets.
Occurrence and Associations
Spessartine is found with other Mn minerals in Mn-rich skarns, low-grade metamorphic rocks, some rare granites and rhyolites, and, occasionally, in pegmatites. Common associated minerals in igneous rocks are quartz, feldspars, and micas.
▪Grossular Ca3Al2Si3O12
Origin of Name
From grossularia, the Latin name for the pale green gooseberry, which is the same color as some grossular.



Hand Specimen Identification
Like all garnets, grossular is hard, commonly vitreous, forms equant crystals and has no cleavage. Grossular is typically rose-colored, pink, red-pink, or olive-green. Its usual occurrence in metacarbonate rocks helps identification. If an unusual color, compositional data may be needed to distinguish it from other garnets.
The grossulars shown in Figures 14.158 and 14.159 display the most common hues for grossular. The emerald-green tsavorite in Figure 14.160 is an unusual but spectacular variety of grossular.
Physical Properties
| hardness | 6.5 |
| specific gravity | 3.56 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/translucent to transparent |
| color | rose, pink, and olive green colors are most common; also brown or yellow |
| streak | white |
Properties in Thin Section
Grossular is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. Isotropic, n = 1.75.
Crystallography
Grossular, like all garnets, is cubic, a = 11.85, Z = 8; space group $ \small {I \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Grossular is characterized by euhedral to subhedral equant grains displaying dodecahedral or trapezohedral faces. Massive occurrences are rare.
Structure and Composition
Grossular is the Al end member of the ugrandite series, Ca3(Fe3+,Al3+,Cr3+)2Si3O12, but natural grossular may contain appreciable amounts of Fe3+ or Cr3+ in solid solution. The structure is the same as other garnets.
Occurrence and Associations
Grossular is found in marbles where it may be associated with calcite, dolomite, quartz, tremolite, diopside, and wollastonite.
Varieties
Hydrogrossular, or hibschite, is the name for grossular in which a substantial amount of Si4+ has been replaced by 4H+. Tsavorite (the green variety seen above in Figure 14.160) gets it green color from trace amounts of vanadium or chromium.
▪Andradite Ca3Fe2Si3O12
Origin of Name
Named after J. B. d’Andrada e Silva (1763–1838), a Portuguese mineralogist.


Hand Specimen Identification
Crystal shape, lack of cleavage, luster, and color identify garnets. The different species can sometimes be inferred by color or association. Andradite‘s color is quite variable but is generally yellow, green or brown. Identifying it with certainty requires analytical data.
Figure 14.161 shows a green gemmy variety of andradite called demantoid. It gets its deep color from trace amounts of Cr in its structure. A small amount of stilbite is also present in the photo. Figure 14.162 shows more typical andradite crystals from Mali.
Physical Properties
| hardness | 7 |
| specific gravity | 3.86 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous, sometimes pearly/translucent to transparent |
| color | yellow, brown, green |
| streak | white |
Properties in Thin Section
Andradite is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. Isotropic, n = 1.87.
Crystallography
Andradite, like all garnets, is cubic, a = 12.05, Z = 8; space group $ \small {I \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Andradite is characterized by euhedral to subhedral equant grains displaying dodecahedral or trapezohedral faces. Massive occurrences are rare.
Structure and Composition
Andradite, the Fe3+ end member of the garnet ugrandite series, may contain appreciable amounts of Al3+ or Cr3+ replacing Fe3+. It has the same structure as other garnets.
Occurrence and Associations
Andradite is found in marbles and occasionally as an accessory mineral in igneous rocks. Typical associated minerals include hedenbergite and magnetite.
Varieties
Melanite is a black variety of andradite. Demantoid (Figure 14.161) is a gemmy green variety of andradite.
▪Uvarovite Ca3Cr2Si3O12
Origin of Name
Named after Count S. S. Uvarov (1785–1855), president of the St. Petersburg Academy in Russia.


Hand Specimen Identification
Crystal shape, lack of cleavage, luster, and hardness identify garnets. The different species can sometimes be inferred by color or association. Uvarovite, like some other chrome minerals, often has a strong emerald green color. But, so do varieties of some other garnet species. Uvarovite‘s occurrence in ultramafic rocks provides hints to its Cr-rich composition, but identifying uvarovite with certainty requires compositional information.
Figure 14.163, above, shows several large uvarovite crystals in a rock from western Finland. Figure 14.164 shows small crystals of the same mineral from the Saranovskii Mine, a classic collecting spot in the central Ural Mountains, Russia.
Physical Properties
| hardness | 7.5 |
| specific gravity | 3.80 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/translucent |
| color | emerald green |
| streak | white |
Properties in Thin Section
Uvarovite is generally clear or very pale in thin section, has high relief, and exhibits no cleavage. Euhedral and subhedral crystals are common. Isotropic, n = 1.85.
Crystallography
Uvarovite, like all garnets, is cubic, a = 12.00, Z = 8;
Habit
Uvarovite is characterized by euhedral to subhedral equant grains displaying dodecahedral or trapezohedral faces. Massive occurrences are rare.
Structure and Composition
Uvarovite, the Cr3+ end member of the garnet ugrandite series, may contain appreciable amounts of Fe3+, or Al3+ replacing Cr3+. It has the same structure as other garnets.
Occurrence and Associations
Uvarovite is a rare mineral found primarily in peridotites and often associated with chrome ore. It is more rarely found in metamorphic rocks. In peridotites, it is typically found with chromite, olivine, pyroxene, and serpentine.
1.5.2 Olivine Group Minerals
|
Olivine Group Minerals |
Olivine, an abundant mineral in mafic and ultramafic igneous rocks, has the general formula (Mg,Fe,Mn)SiO4. Its structure is similar to that of garnet: isolated SiO4 tetrahedra are linked by divalent cations in octahedral coordination. Complete solid solution exists between the important end members forsterite (Mg-olivine) and fayalite (Fe-olivine), and, less important, tephroite (Mn-olivine). Limited solid solution toward a Ca2SiO4 end member is also possible, but the rare mineral larnite, with composition Ca2SiO4, does not have the olivine structure. Monticellite, CaMgSiO4, is grouped with the olivines, but because of its highly distorted structure is not considered a true olivine.
For more general information about the olivine group, see the olivine section in Chapter 5.
▪Forsterite Mg2SiO4
Origin of Name
Named after Jacob Forster, a scientist and founder of Heuland Cabinet.



Hand Specimen Identification
Olivine is distinguished by its glassy luster, conchoidal fracture, and usually olive-green color. Association and alteration to serpentine help identification sometimes. Olivines are occasionally confused with epidote or green pyroxene.
Forsterite is the name of end-member Mg2SiO4–olivine and also a general name used for any Mg-rich olivine. Distinguishing forsterite from other olivines is usually based on color (forsterite is often green but may be white if it contains little Fe) but certain identification requires optical or X-ray data.
Figure 14.165 shows the most common occurrence of visible forsterite crystals – as phenocrysts in a volcanic rock such as basalt. Figure 14.166 shows millimeter-sized forsterite phenocrysts that were removed from a basalt. Figure 14.167 is a photo of euhedral forsterite crystal that came from olivinite dikes on St. Johns Island in the Red Sea, Egypt.
Physical Properties
| hardness | 6.5 |
| specific gravity | 3.2 |
| cleavage/fracture | poor (010) and (100)/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | varies, white or green, less commonly yellow |
| streak | white |
Properties in Thin Section
Mg-rich olivines are colorless in thin section. Index of refraction and birefringence are high. Poor cleavage, irregular fracture, often equant grains, relatively high birefringence, and alteration to serpentine or chlorite help identification. Biaxial (+), α = 1.635 , β = 1.651, γ = 1.670, δ = 0.035, 2V = 85° to 90°.
Crystallography
Forsterite and other olivine minerals are orthorhombic, a = 4.78, b = 10.28, c = 6.00, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Rare euhedral olivine crystals are combinations of prisms and dipyramids, often having a tabular or lozenge shape. Granular forms that resemble green sand, or embedded grains, are common.
Structure and Composition
In olivine, isolated SiO4 tetrahedra are linked by MgO6 octahedra. Complete solid solution exists between forsterite (Mg2SiO4), fayalite (Fe2SiO4), and tephroite (Mn2SiO4). Minor Ca or Ni may also be present as replacement for Mg.



Occurrence and Associations
Forsterite, a primary mineral in many mafic and ultramafic rocks, is typically associated with pyroxenes, plagioclase, spinel, garnet, and serpentine. Figures 14.168 and 14.169 show two examples of forsterite in dunites, olivine-dominated ultramafic rocks.
Less commonly, forsterite is found as a metamorphic mineral. For example, Figure 14.170 shows green forsterite crystals in a marble. Even less commonly, forsterite is found in young sediments

Varieties
Peridot (Figure 14.171) is a gemmy green transparent variety of forsterite.
Related Minerals
The principal olivine end members are forsterite, fayalite, and tephroite. Olivine is isostructural with chrysoberyl, BeAl2O4. Minerals with similar but not identical structures include monticellite (CaMgSiO4), sinhalite (MgAlBO4), larnite (Ca2SiO4), and kirschsteinite (CaFeSiO4).
▪Fayalite Fe2SiO4
Origin of Name
Named after Fayal Island of the Azores, where fayalite was once found.


Hand Specimen Identification
Common olivine is distinguished by its glassy luster, conchoidal fracture, and usually olive-green color. In the absence of compositional data, we assume any green olivine to be Mg-rich, and thus call it forsterite. Fayalite (Fe-rich olivine), in contrast, is often in various shades of brown or yellow. Certain identification, however requires X-ray or optical data.
Figure 14.172 shows a small single crystal of brown fayalite, and Figure 14.173 shows a mass of fayalite crystals that range from brown to green in color.
Physical Properties
| hardness | 6.5 |
| specific gravity | 4.4 |
| cleavage/fracture | poor (010) and (100)/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | brown, yellow, or greenish-yellow |
| streak | white or yellow |
Properties in Thin Section
Fe-rich olivines are pale yellow or green in thin section and may be weakly pleochroic. Index of refraction and birefringence are high. Poor cleavage, often equant grains, and alteration to serpentine or chlorite help identification. Biaxial (-), α = 1.827 , β = 1.877, γ = 1.880, δ = 0.053, 2V = 47° to 54°.
Crystallography
Fayalite is orthorhombic, a = 4.81, b = 10.61, c = 6.11, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Rare euhedral fayalite crystals display combinations of prisms and dipyramids, often having a tabular or lozenge shape. Subhedral or anhedral embedded grains are common.
Structure and Composition
Fayalite structure is the same as that of other olivines: isolated SiO4 tetrahedra are linked by FeO6 octahedra. Complete solid solution exists between fayalite (Fe2SiO4), forsterite (Mg2SiO4), and tephroite (Mn2SiO4). Minor Ca or Ni may also be present as replacement for Fe.
Occurrence and Associations
Fayalite, less common than Mg-rich olivine, is found in some Fe-rich granitic igneous or metamorphic rocks.
Related Minerals
The principal olivine end members are forsterite, fayalite, and tephroite. Many other minerals have identical or related structures (see forsterite).
▪Monticellite CaMgSiO4
Origin of Name
Named after Italian mineralogist Teodoro Monticelli (1759–1846).



Hand Specimen Identification
Occurrence in high-temperature metacarbonates, association with other metacarbonate minerals and calcite, common brown color, conchoidal fracture, and habit help identify monticellite. It is difficult to distinguish from other olivine minerals without optical or X-ray data.
Figures 14.174 and 14.175 show photos of monticellite from the classic collecting site in Crestmore, California. In both, blue calcite accompanies the monticellite. Figure 14.176 shows a single crystal of transparent very light tan monticellite from a quarry near Koblenz, Germany. The monticellite crystal is on white calcite.
Physical Properties
| hardness | 5.5 |
| specific gravity | 3.15 |
| cleavage/fracture | poor (010) and (100)/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white, gray, or green |
| streak | white |
Properties in Thin Section
Monticellite is similar to olivine but has greater 2V. Biaxial (-), α = 1.645 , β = 1.655, γ = 1.665, δ = 0.020, 2V = 72° to 82°.
Crystallography
Monticellite is orthorhombic, a = 4.82, b = 11.08, c = 6.38, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Crystals tend to be subequant combinations of prisms and dipyramids. Monticellite is usually embedded grains or massive patches in a carbonate-rich host.
Structure and Composition
The structure of monticellite is similar to that of olivine, but the mismatch in size between Ca and Mg leads to some slight differences (see forsterite structure). Fe may substitute for Mg, leading to solid solutions with kirschsteinite, CaFeSiO4. Minor Al and Mn may also be present.
Occurrence and Associations
A rare mineral, monticellite is found in skarns and, less commonly, in regionally metamorphosed rocks. Associated minerals include calcite, forsterite, åkermanite, merwinite, and tremolite. Very minor occurrences have been reported from ultramafic igneous rocks.
Related Minerals
The true olivines (forsterite, fayalite, tephroite) are closely related to monticellite. Kirschsteinite, CaFeSiO4, is isostructural with monticellite. Minerals with similar but not identical structures include sinhalite, MgAl(BO4), and larnite, Ca2SiO4.
1.5.3 Humite Group Minerals
|
Humite Group Minerals |
The Humite Group contains about 10 minerals; the four Mg-humites listed in the blue box are the most important. Other minerals of the group contain Mn, Ca, or Zn in place of some or all the Mg.
All minerals of the humite group are isolated tetrahedral silicates with structural similarity to olivine. Their general formula is nMg2SiO4•Mg(OH,F)2, where n is 1, 2, 3, and 4, respectively, for norbergite, chondrodite, humite, and clinohumite. Chondrodite is the most common of the humite minerals. Humite is relatively rare; only the other three are described below. These minerals have similar compositions and share many properties, making it difficult to distinguish one from another.
▪Norbergite Mg3SiO4(OH,F)2
Origin of Name
Named after the type locality at Norberg, Sweden.


Hand Specimen Identification
The most common members of the humite group (norbergite, chondrodite, and clinohumite) cannot be distinguished without optical or X-ray data. They are usually identified by association, light color, and their form. They may be difficult to distinguish from olivine. The photos shown in Figures 14.177 and 14.198 show typical brown norbergite. In Figure 14.178, calcite accompanies the norbergite.
Physical Properties
| hardness | 6.5 |
| specific gravity | 3.16 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white, yellow, brown, red |
| streak | white |
Properties in Thin Section
Humite group minerals are biaxial (+). They may resemble olivine in thin section, but most olivines are biaxial (-). Additionally, humite-group minerals have lower birefringence than olivine. Norbergite is biaxial (+), α = 1.561 , β = 1.570, γ = 1.587, δ = 0.026, 2V = 44° to 50°.
Crystallography
Norbergite is orthorhombic, a = 4.70, b = 10.22, c = 8.72, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Norbergite crystals, usually found as isolated grains, are variable and display many forms. Highly modified orthorhombic or pseudoorthorhombic shapes are common.
Structure and Composition
Norbergite‘s structure consists of alternating layers of forsterite and brucite composition. Norbergite is usually close to end-member composition, although F:OH ratios are variable. Some Fe may replace Mg.
Occurrence and Associations
Norbergite is a rare mineral found in metamorphosed carbonate rocks. Associated minerals include calcite, dolomite, phlogopite, diopside, spinel, wollastonite, grossular, forsterite, and monticellite.
▪Chondrodite Mg5(SiO4)2(OH,F)2
Origin of Name
From the Greek word chondros meaning “grain,” referring to this mineral’s occurrence as isolated grains.



Hand Specimen Identification
The members of the humite group are usually identified by association, light color, and form. Chondrodite is difficult to distinguish from other humite group minerals and from olivine, even when viewed in thin section with an optical microscope.
Figure 14.179 shows a single crystal of euhedral chondrodite. But, this mineral more commonly occurs as disseminated subhedral to anhedral grains in marbles; Figures 14.180 and 14.181 are two examples of chondrodite-bearing marbles.
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 3.16 to 3.26 |
| cleavage/fracture | poor (100)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white to yellow |
| streak | white |
Properties in Thin Section
Chondrodite and other humite group minerals resemble olivines in thin section, but most olivines are biaxial (-) instead of (+), and humites have lower birefringence. Biaxial (+), α = 1.60, β = 1.62, γ = 1.63, δ = 0.03, 2V = 60° to 90°.
Crystallography
Chondodrite is monoclinic, a = 4.73, b = 10.27, c = 7.87, β = 109.1°, Z = 2; space group $ \small{P \frac{2_1}{b}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Usually found as isolated grains, chondrodite crystals are variable and display many forms. Highly modified orthorhombic or pseudoorthorhombic crystals, with or without {001} twinning, are common.
Structure and Composition
Chondodrite‘s structure consists of layers of forsterite and brucite composition. Chondrodite contains two forsterite layers for each brucite layer. F:OH ratios are variable. Some Fe may replace Mg, and Ti content can be substantial.
Occurrence and Associations
Like norbergite, chondrodite is a rare mineral found in metamorphosed carbonate rocks. Associated minerals include calcite, dolomite, phlogopite, diopside, spinel, wollastonite, grossular, forsterite, and monticellite. Chondrodite has also been found in a few rare carbonatites.
▪Clinohumite Mg9(SiO4)4(OH,F)2
Origin of Name
Named after English mineralogist Sir Abraham Hume (1749–1839).


Hand Specimen Identification
The members of the humite group (norbergite, chondrodite, and clinohumite) cannot be distinguished from each other, and sometimes from olivine, without optical or X-ray data. They are usually identified by association, light color, and form. The photos show two views of clinohumite from Tajikistan. The stones in the photo on the left are advertised as gem quality rough; a parcel of 100 pieces was selling for $700 in 2022.
Physical Properties
| hardness | 6 |
| specific gravity | 3.21 to 3.35 |
| cleavage/fracture | poor (100)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white to yellow |
| streak | white |
Properties in Thin Section
Clinohumite and other humite group minerals resemble olivines in thin section, but most olivines are biaxial (-), and humites have lower birefringence. Clinohumite is biaxial (+), α = 1.63 , β = 1.64, γ = 1.59, δ = 0.03 to 0.04, 2V = 73° to 76°.
Crystallography
Clinohumite is monoclinic, a = 4.75, b = 10.27, c = 13.68, β = 100.8°, Z = 2; space group $ \small{P \frac{2_1}{b}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Usually found as isolated grains, clinohumite crystals are variable and display many forms. Highly modified pseudoorthorhombic crystals with or without {001} twinning are common.
Structure and Composition
Clinohumite‘s structure is similar to the other humite group minerals (see chondrodite structure) except that the ratio of forsterite: brucite layers is 4:1. Some Fe may replace Mg, and F:OH ratios are variable. Ti is almost always present in small amounts.
Occurrence and Associations
The most significant occurrences of clinohumite are in metamorphosed carbonate rocks, similar to other humite-group minerals.

Varieties
Titanoclinohumite, an especially Ti-rich variety, has been reported from a few rare serpentinites and gabbros. Figure 14.184 shows red titanoclinohumite in a rock that also contains light-green clinochlore.
1.5.4 Aluminosilicates
|
Aluminosilicate Group Minerals |
The aluminosilicate polymorphs vary little from stoichiometric Al2SiO5 composition. All are isolated tetrahedral silicates but have distinctly different structures. In kyanite all the Al is in 6-fold coordination, in andalusite half is in 5-fold coordination and half is in 6-fold coordination, and in sillimanite half is in 4-fold coordination and half is in 6-fold coordination. The aluminosilicates are important minerals in pelitic metamorphic rocks. As discussed in Chapter 8, the presence of a particular polymorph indicates a general range of pressure-temperature at which the rock must have formed. For this reason, and because they are relatively common, the aluminosilicates are key metamorphic index minerals.
▪Kyanite Al2SiO5
Origin of Name
From the Greek word kyanos, meaning “blue.”

Hand Specimen Identification
Kyanite is brittle, forms splintery/bladed crystals, and is easily cleaved into acicular fragments. It is almost always some shade of blue, and there are few other blue minerals that form long bladed crystals. A blue color and occurrence in metamorphosed pelites or quartzites are adequate to identify it in most cases. Figure 14.185 shows blades of kyanite in a metaquartzite. Figure 3.64 shows other examples of splintery kyanite blades.
Physical Properties
| hardness | 5 to 7 |
| specific gravity | 3.60 |
| cleavage/fracture | two prominent: perfect (100), good (010)/uneven |
| luster/transparency | vitreous, sometime pearly/transparent to translucent |
| color | typically light to dark blue, may be white |
| streak | white |
Properties in Thin Section
Kyanite is typically colorless in thin section, but may be weakly blue and pleochroic. High relief, low birefringence, and excellent cleavage aid identification. It may be confused with sillimanite or andalusite, but sillimanite has a small 2V, and andalusite has parallel extinction. Kyanite is biaxial (-), α = 1.712 , β = 1.720, γ = 1.728, δ = 0.016, 2V = 82° to 83°.
Crystallography
Kyanite is triclinic, a = 7.10, b = 7.74, c = 5.57, α = 90.08°, β = 101.03°, γ = 105.73°, Z = 4; space group $ \small{P\overline{1}} $; point group $ \small{\overline{1}}$.
Habit
Kyanite is usually in long blade-shaped or tabular crystals, sometimes forming parallel or radiating aggregates.
Structure and Composition
In kyanite, chains of AlO6 octahedra are linked by additional AlO6 octahedra and by SiO4 tetrahedra. Kyanite is always near to Al2SiO5 composition; it may contain very minor Fe, Mn, or Cr.

Occurrence and Associations
Kyanite is primarily a metamorphic mineral found in medium- and high-pressure schists and gneisses. Figure 14.186 is a photo of a typical kyanite schist. Common associated minerals are quartz, feldspar, mica, garnet, corundum, and staurolite. Kyanite is also (rarely) found in aluminous eclogites and other rocks of deep origin.
Related Minerals
Kyanite has two polymorphs, andalusite and sillimanite.
▪Andalusite Al2SiO5
Origin of Name
Named for Andalusia, a province of Spain.



Hand Specimen Identification
Andalusite is found in metapelites. It is recognized primarily by crystal shape, prismatic habit (Figure 14.187), and association. Hardness and nearly square (diamond) cross sections help identify andalusite. A variety called chiastolite displays a “maltese cross” pattern that is diagnostic. Figures 14.188 and 14.189 show examples of chiastolite. Andalusite is sometimes confused with staurolite (which may have a similar habit and is also common in metapelites) or scapolite.
Physical Properties
| hardness | 7.5 |
| specific gravity | 3.18 |
| cleavage/fracture | rarely seen, good {110}, poor (100)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | brown to red |
| streak | white |
Properties in Thin Section
Usually clear in thin section, andalusite may be weakly colored and pleochroic. Euhedral crystals with a square outline, sometimes showing penetration twins or a maltese cross pattern, are diagnostic. Andalusite is length fast and has a high 2V. Biaxial (-), α = 1.632 , β = 1.640, γ = 1.642, δ = 0.010, 2V = 75° to 85°.
Crystallography
Andalusite is orthorhombic, a = 7.78, b = 7.92, c = 5.57, Z = 4; space group $ \small{P \frac{2_1}{n} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Stubby to elongate square prisms characterize andalusite. Individual crystals may be rounded. Massive and granular forms are also known.
Structure and Composition
Andalusite consists of chains of AlO6 octahedra parallel to the c-axis, linked by SiO4 tetrahedra and by AlO5 polyhedra. Andalusite is usually close to Al2SiO5 in composition. Small amounts of Mn, Fe, Cr, and Ti may be present.

Occurrence and Associations
Andalusite is a metamorphic mineral characteristic of relatively low pressures. It occurs in pelitic rocks, often associated with cordierite, sillimanite, kyanite, garnet, micas, and quartz. Figure 14.190 shows andalusite crystals in a muscovite schist from near Dublin, Ireland mica schist. The largest crystals are several centimeters long.
Varieties
Chiastolite is a variety of andalusite that has a square cross section (001) displaying a maltese cross pattern. The pattern results from carbonaceous impurities included during crystal growth.
Related Minerals
Andalusite has two polymorphs, sillimanite and kyanite.
▪Sillimanite Al2SiO5
Origin of Name
Named after Benjamin Silliman (1779–1864), a chemistry professor at Yale University.



Hand Specimen Identification
Sillimanite is found in high-grade pelites, in the form of fine needles (Figure 14.191), coarse blades (Figure 14.192), or prismatic acicular crystals (Figure 14.193) with square cross sections. The three figures here show examples. Aggregates may be masses or sprays. Sillimanite is occasionally confused with anthophyllite. Crystal form and habit, and occurrence in metapelitic rocks generally serve to identify this mineral.
Physical Properties
| hardness | 6 to 7 |
| specific gravity | 3.23 |
| cleavage/fracture | perfect but rarely seen (010)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | white to brown |
| streak | white |
Properties in Thin Section
Sillimanite typically forms needles, often in masses or mats, with square cross sections showing one good diagonal cleavage. It has high relief, small 2V, (+) optic sign, and is length slow. Biaxial (+), α = 1.658 , β = 1.662, γ = 1.680, δ = 0.022, 2V = 20° to 30°.
Crystallography
Sillimanite is orthorhombic, a = 7.44, b = 7.60, c = 5.75, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2}{m} \frac{2}{n}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Long, slender prisms, needles, or fibers are common habits for sillimanite. Subparallel aggregates and sprays are typical. Fine-grained fibrous mats are also common.
Structure and Composition
Sillimanite‘s structure consists of chains of AlO6 octahedra, parallel to the c-axis,fa linked by SiO4 and AlO4 tetrahedra. Composition is always close to stochiometric Al2SiO5; small amounts of Fe may be present.


Occurrence and Associations
Sillimanite is the high-temperature Al2SiO5 polymorph, found in high-grade pelites associated with garnet, cordierite, spinel, hypersthene, orthoclase, biotite, and quartz. Often, individual sillimanite needles are hard to see but give a metamorphosed rock a foliated texture, like the example rocks in Figures 14.194 and 14.195. If you enlarge the photos, you can (just barely) make out the sillimanite needles.
Varieties
Fibrolite is the name given to fine-grained fibrous masses of sillimanite. Fibrolite is generally only visible in thin section.
Related Minerals
Sillimanite has two polymorphs: andalusite and kyanite.
1.5.5 Other Isolated Tetrahedral Silicates
|
Other Isolated Tetrahedral Silicates |
Besides those already listed, a number of other isolated tetrahedral silicates are common and important minerals. Although they have structures based on isolated SiO4 tetrahedra, they do not fit into any of the previously discussed structural groups.
Staurolite and chloritoid are important metamorphic minerals in rocks rich in Fe and Al. Titanite and zircon are common accessory minerals in silicic igneous rocks and in many metamorphic rocks. Topaz is most commonly found in pegmatites and hydrothermal veins associated with granites and other silicic igneous rocks.
▪Staurolite Fe2Al9Si4O23(OH)
Origin of Name
From the Greek word stauros, meaning “cross,” in reference to its cruciform twins.


Hand Specimen Identification
Staurolite is often easily recognized by its brown color, characteristic penetration twins, and prismatic crystals with a diamond-shaped cross section. These two photos show examples.
Staurolite is sometimes confused with andalusite, in part because of its typical brown color and occurrence in metapelites, but has different habit. Pyroxene, tourmaline, titanite, and amphibole may look superficially like staurolite.
Physical Properties
| hardness | 7 to 7.5 |
| specific gravity | 3.75 |
| cleavage/fracture | poor {010}/subconchoidal |
| luster/transparency | vitreous, sometimes resinous/translucent |
| color | brown to gray or black |
| streak | white |
Properties in Thin Section
Staurolite may be clear to yellow or light brown in thin section, often pleochroic. Birefringence is low; maximum colors are first-order yellow. Anhedral to euhedral porphyroblasts, often exhibiting a “sieve” structure due to quartz inclusions, or showing penetration twins, are common. Biaxial (+), α = 1.740 , β = 1.744, γ = 1.753, δ = 0.013, 2V = 80° to 88°.
Crystallography
Staurolite is monoclinic, a = 7.82, b = 16.52, c = 5.63, β = 90.0°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Staurolite is usually found as prismatic crystals, often flattened in one direction and having several terminating forms. Massive varieties are rare. Penetration twins are common, often resulting in perfect cruciform crosses, sometimes called fairy crosses (Figure 14.197, above).
Structure and Composition
Staurolite structure is closely related to that of kyanite. Layers of Al2SiO5, including AlO6 octahedra in chains, alternate with layers of Fe(OH)2. Pure end-member Fe-staurolite does not exist in nature; Mg is always present, replacing up to 35% of the Fe. Small amounts of Ti and Mn are generally present as well. Water content is slightly variable.

Occurrence and Associations
Staurolite is a metamorphic mineral common in medium- to high-grade metamorphic rocks. Associated minerals include kyanite, garnet, chloritoid, micas, and tourmaline. The rock seen in Figure 14.198 is a typical staurolite schist that contains coarse crystals of staurolite in a sea of sparkly mica.
▪Chloritoid (Fe,Mg)Al2SiO5(OH)2
Origin of Name
Named for its resemblance to chlorite.



Hand Specimen Identification
Green color, cleavage, occurrence in metapelites, and association with other pelitic minerals help identify chloritoid. In many metamorphic rocks it appears as small dark grains, green to black, or as patches in a micaceous matrix – and is hard to see. Thin sections may be required to distinguish it from chlorite, biotite, or stilpnomelane. Figure 14.199 shows a typical example. The photos in Figures 14.200 and 14.201 show exceptional coarser euhedral specimens.
Physical Properties
| hardness | 6.5 |
| specific gravity | 3.5 |
| cleavage/fracture | poor{110}/uneven |
| luster/transparency | pearly, sometimes vitreous/translucent |
| color | dark green or gray/black |
| streak | gray |
Properties in Thin Section
Chloritoid is typically colorless to green in thin section and may exhibit pleochroism in various shades of green, yellow, or blue. It has high relief and anomalous interference colors and is frequently twinned. Chlorite looks superficially like chloritoid but has significantly lower RI and relief and a smaller 2V. Biaxial (+), α = 1.715 , β = 1.720, γ = 1.725, δ = 0.010, 2V = 45° to 65°.
Crystallography
Chloritoid is monoclinic, a = 9.52, b = 5.47, c = 18.19, β = 101.65°, Z = 6; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Coarse masses or thin scales are typical for chloritoid; individual crystals are rare. Tabular crystals are platy and foliated with common {001} twinning.
Structure and Composition
The chloritoid structure is layered. Alternating brucite-like and corundum-like layers, perpendicular to the c-axis, are linked by SiO4 tetrahedra and hydrogen bonds. Chloritoid is not a layered silicate, like chlorite, because the SiO4 tetrahedra do not share oxygen. Chloritoid is generally Fe-rich, but Fe:Mg ratios are variable; end members are not found in nature. Some Mn may be present.
Occurrence and Associations
Chloritoid is common in low- or medium-grade Fe-and Al-rich schists. Associated minerals include quartz, feldspars, muscovite, chlorite, staurolite, garnet, andalusite, and kyanite. In some rare high-pressure metamorphic rocks, it occurs with glaucophane and other blueschist minerals.
Varieties
Ottrelite is Mn-rich chloritoid; carboirite is Ge-containing chloritoid.
Related Minerals
Several different polytypes and polymorphs have been described.
▪Titanite (Sphene) CaTiSiO5
Origin of Name
This mineral’s name refers to its titanium content. Its older name, sphene, refers to its crystal (sphenoid) shape.


Hand Specimen Identification
Adamantine or vitreous luster, brown or green color, and diamond or wedge-shaped crystals help identify titanite. You can see the diagnostic shapes in some of the crystals in these two photographs.
In most rocks, titanite is so fine grained that it is hard to pick out without a thin section and microscope. Coarse crystals may occasionally be confused with staurolite and zircon, but titanite is softer; or with sphalerite, but titanite is harder.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 3.50 |
| cleavage/fracture | good prismatic {110}, poor (100)/uneven |
| luster/transparency | adamantine or vitreous/transparent to translucent |
| color | usually brown, less commonly gray-green, yellow-green, or black |
| streak | white |
Properties in Thin Section
Very high relief and birefringence and distinctive wedge- or diamond-shaped crystals characterize titanite. Biaxial (+), α = 1.86 , β = 1.93, γ = 2.10, δ = 0.15, 2V = 23° to 50°.
Crystallography
Titanite is monoclinic, a = 6.56, b = 8.72, c = 7.44, β = 119.72°, Z = 4; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Sphenoidal crystals, tabular with a wedge or diamond shape in cross section, are typical. Less commonly, titanite is massive or lamellar. Titanite is normally fine grained but occasionally occurs as large crystals.
Structure and Composition
Titanite‘s structure contains TiO6 octahedra and SiO4 tetrahedra that share corners, forming distorted chains parallel to a. Ca is in 7-fold coordination, in large holes between the Ti- and Si-polyhedra. Many elements may substitute in titanite; the rare earth elements are especially important.
Occurrence and Associations
Titanite may be very fine grained and is an often overlooked, but common, widespread accessory mineral. In many rocks it is the only Ti mineral present. It is found in many igneous rocks, especially silicic to intermediate ones, and many metamorphic rocks. It also has been found in some limestones and a few rare clastic sediments. Associated minerals include just about all the important rock forming minerals, including pyroxene, amphibole, feldspar, and quartz.

Varieties
Greenovite (Figure 14.204) is the name given to red or pink titanite.
Related Minerals
Titanite is isostructural with tilasite, CaMg(AsO4)F; malayaite, CaSn(SiO4)O; and with fersmantite, (Ca,Na)4(Ti,Nb)2Si2O11(F,OH)2. Other related titanium minerals include perovskite, CaTiO3; benitoite, BaTiSi3O9; and neptunite, KNa2Li(Fe,Mn)2Ti2O(Si4O11)2.
▪Topaz Al2SiO4(F,OH)2
Origin of Name
Named after Topazion, an island in the Red Sea.



Hand Specimen Identification
Orthorhombic form, hardness (H=8), one good cleavage, color, and luster identify topaz. It may be confused with quartz but is orthorhombic, not hexagonal. The very rare mineral danburite, Ca(B2Si2O8), is similar to topaz in form and other properties, and sometimes cannot be distinguished without chemical analysis.

Topaz has many different appearances but when euhedral can appear as well-formed orthorhombic crystals. The clear crystals shown in Figure 14.205, and the yellow ones in Figures 14.206 and 14.207 are most typical. Colorful and gemmy topaz is especially valued by mineral collectors and gemologists –Figure 14.208 shows an example of pink gem topaz from Pakistan.
Physical Properties
| hardness | 8 |
| specific gravity | 3.5 to 3.6 |
| cleavage/fracture | one perfect (001)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | generally colorless, but sometimes variable hues |
| streak | white |
Properties in Thin Section
Topaz is typically colorless in thin section and has low birefringence. It resembles quartz and apatite but has one perfect cleavage and higher relief than quartz, is biaxial, and is length slow. Biaxial (+), α = 1.61 , β = 1.61, γ = 1.62, δ = 0.01, 2V = 48° to 65°.
Crystallography
Topaz is orthorhombic, a = 4.65, b = 8.80, c = 8.40, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Typical topaz crystals are orthorhombic prisms, terminated by dipyramids and pinacoids in combination. Prism faces show striations. Cross sections may be square, rectangular, diamond shaped, or octagonal. Coarse- or fine-grained masses are also common.
Structure and Composition
The structure of topaz consists of chains, parallel to the c-axis, containing pairs of edge-sharing Al(OH,F)6 octahedra alternating with SiO4 tetrahedra. F and OH content are variable, but F:OH ratio is usually in excess of 6:1. No other significant substitutions are known.
Occurrence and Associations
Topaz is a late-stage igneous or hydrothermal mineral. It is an accessory in granite, rhyolite, and granitic pegmatites and may be found in contact aureoles adjacent to silicic plutons. It is often associated with lithium and tin mineralization. Associated minerals include quartz, feldspar, muscovite, tourmaline, fluorite, cassiterite, apatite, and beryl. It may be very fine-grained and easily overlooked in many kinds of rocks.
Related Minerals
Euclase, BeAl(SiO4)(OH), is isotypical with topaz.
▪Zircon ZrSiO4
Origin of Name
From the Persian zar (“gold”) and gun (“color”).



Hand Specimen Identification
Zircon can sometimes be identified by its hardness, brown to brown-red color, and tetragonal crystal shape. Tetragonal symmetry shows in Figures 14.209 and 14.210. But when the symmetry is not evident, like the specimens in Figure 14.211, zircon can be confused with other red-brown minerals such as titanite or rutile.
Physical Properties
| hardness | 7.5 |
| specific gravity | 4.68 |
| cleavage/fracture | poor (100), poor {101}/conchoidal |
| luster/transparency | adamantine, sometimes resinous/transparent to translucent |
| color | red or red-brown is typical, also gray, green, or colorless |
| streak | white |
Properties in Thin Section
In thin section, zircon is normally colorless but may be pale yellow or brown and faintly pleochroic. Grains are typically small, exhibiting very high relief and birefringence. Pleochroic halos around grains are due to decay of radioactive elements. Uniaxial (+), ω = 1.99, ε = 1.93, δ = 0.06.
Crystallography
Zircon is tetragonal, a = 6.59, c = 5.99, Z = 4; space group $ \small{I \frac{4_1}{a} \frac{2}{m} \frac{2}{d}} $; point group $ \small{ \frac{4}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Zircon typically is found as square prisms, pyramids/dipyramids, or as combinations of the two. Rounded grains are also common.
Structure and Composition
The zircon structure contains SiO4 tetrahedra sharing corners or edges with distorted cubic polyhedra containing Zr. Zircon is generally close to end-member composition, but frequently contains small amounts of Al, Fe, Mg, Ca, rare earths, and water.
Occurrence and Associations
Zircon is a common and widespread accessory mineral in igneous rocks, especially silicic ones. It is found in many metamorphic rocks and is common in sediments and sedimentary rocks. It is an important mineral for some kinds of radiometric dating. Grain size in rocks can be very small, so zircon is easily overlooked.
Varieties
Zircon is often metamict (structurally damaged by the decay of radioactive elements in its structure), causing variation in color and optical properties.
Related Minerals
Zircon has a number of isotypes, including thorite, (Th,U)(SiO4); xenotime, Y(PO4); and huttonite, ThSiO4. Baddeleyite, ZrO2, is another Zr-rich mineral.
1.6 Paired Tetrahedral Silicates
|
Paired Tetrahedral Silicates |
Mineralogists often group lawsonite, epidote, clinozoisite, and vesuvianite because they all contain pairs of SiO4 tetrahedra sharing a single bridging oxygen. Åkermanite and gehlenite, rare minerals belonging to the melilite group, are also paired tetrahedral silicates but are not considered in detail in this book.
In lawsonite all silica tetrahedra are paired. However, in epidote, clinozoisite, and vesuvianite, structures are more complicated because some SiO4 tetrahedra are unpaired. For this reason, these three minerals are sometimes not considered to be “true” paired tetrahedral silicates.
For more general information about paired tetrahedral silicates Section 6.4.6 in Chapter 6.
▪Lawsonite CaAl2Si2O7(OH)2•H2O
Origin of Name
Named after A. C. Lawson (1861–1952), a professor at the University of California.


Hand Specimen Identification
Occurrence in high-pressure metamorphic rocks, hardness, light color, and bladed or blocky character help identify lawsonite, but it is often very fine grained. The two photos seen here show coarse lawsonite crystals from the Franciscan terrane of California.
Physical Properties
| hardness | 8 |
| specific gravity | 2.1 |
| cleavage/fracture | perfect (100) and (010), fair {101}/uneven |
| luster/transparency | greasy, vitreous/transparent |
| color | typically gray, white, pale blue, or colorless |
| streak | white |
Properties in Thin Section
Lawsonite is usually colorless and exhibits high relief and interference colors no higher than first-order red. Biaxial (+), α = 1.665 , β = 1.674, γ = 1.685, δ = 0.020, 2V = 76° to 86°
Crystallography
Lawsonite is orthorhombic, a = 8.90, b = 5.76, c = 13.33, Z = 4; space group $ \small{C \frac{2}{c} \frac{2}{m} \frac{2}{m}} $; point group $ \small{\frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Lawsonite may form tabular or prismatic crystals. Simple or lamellar twins are common.
Structure and Composition
Lawsonite is the only relatively common silicate in which all (SiO4) tetrahedra are paired, thus producing Si2O7 groups. The Si2O7 groups link Al(O,OH) octahedra; Ca occupies holes between the Si2O7 groups and the octahedra. Minor amounts of Ti, Fe, Mg, Na, and K may be present, but no major solid solutions are known.
Occurrence and Associations
Lawsonite is a metamorphic mineral typical of the blueschist facies. Associated high-pressure minerals include glaucophane, jadeite, pumpellyite, or aragonite. Other associated minerals include chlorite, plagioclase, titanite, quartz, and epidote.
Related Minerals
Hemimorphite, Zn4(Si2O7)(OH)2•H2O, and ilvaite, CaFe3O(Si2O7)(OH), are other paired tetrahedral silicates in which all SiO4 tetrahedra are paired. Other minerals usually considered in the same group are epidote, Ca2(Al,Fe)3Si3O12(OH); clinozoisite, Ca2Al3Si3O12(OH); piemontite, Ca2(Al,Mn)3Si3O12(OH); allanite, (Ca,Ce)2(Al,Fe,Mg)3Si3O12(OH); and vesuvianite, Ca10(Mg,Fe)2Al4Si9O34(OH,F)4.
▪Epidote Ca2(Al,Fe)3Si3O12(OH)
Origin of Name
From the Greek word epididonai, meaning “increase,” referring to the base of an epidote prism, one side of which is longer than the other.
Hand Specimen Identification
Epidote is most easily identified by its pistachio-green color (although some epidote is darker green), prismatic habit, and sometimes by its occurrence as an alteration product or accessory mineral. It is often very fine-grained or massive. The photo in Figure 14.214 below shows a rock made entirely of fine-grained epidote. The other photos show coarser specimens exhibiting well-developed prismatic character.
Physical Properties
| hardness | 6 to 7 |
| specific gravity | 3.4 to 3.5 |
| cleavage/fracture | perfect (001), poor (100)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | pistachio-green, less commonly yellow-green to dark green or black |
| streak | white |
Properties in Thin Section
In thin section, epidote has high relief and is usually colorless, but may be light green or pink and pleochroic, depending on composition. Interference colors range up to third order as Fe content increases. Football-shaped grains with concentric interference color rings are diagnostic of epidote. Epidote is biaxial (-), a = 1.71, to 1.75 , β = 1.72 to 1.78, γ = 1.73 to 1.80, δ = 0.01 to 0.05, 2V = 90° to 115°
Crystallography
Epidote is monoclinic, a = 8.98, b = 5.64, c = 10.22, β = 115.4°, Z = 2; space group $ \small{P \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Epidote crystals are usually prismatic elongated parallel to b, with faces showing striations. Fibrous or acicular crystals are common, too. Granular, massive, and fibrous aggregates are common.
Structure and Composition
Epidote is usually considered a paired tetrahedral silicate, but contains both paired and unpaired (SiO4) tetrahedra. Chains of edge-sharing Al(O,OH)6 octahedra, are linked by Si2O7 and SiO4 groups. Ca occupies large sites between the various groups. A complete solid solution exists between Fe-epidote, Ca2Fe3Si3O12(OH) and clinozoisite, Ca2Al3Si3O12(OH). Limited substitution exists between epidote and piemontite, Ca2(Mn,Al)3Si3O12(OH). Cr, Pb, V, Sr, Sn, and rare earths may be present in small amounts.
Occurrence and Associations
Epidote is a common and widespread mineral, characteristic of low- to medium-grade metabasites and marbles. Associated minerals include actinolite, chlorite, and albite in mafic rocks and diopside, grossular, and vesuvianite in marbles. Epidote is also produced by alteration of feldspar, pyroxene, and amphibole.
Varieties
Pistacite is a name sometimes used for pistachio green epidote. Allanite is an epidote mineral rich in rare earth elements. Hancockite is a pink Pb-rich variety of epidote. Sausserite is a name given to fine-grained epidote produced by alteration of plagioclase. Sr-rich epidote is called epidote-(Sr).
Related Minerals
Epidote is similar to other minerals of the epidote group. The group includes epidote, clinozoisite, allanite, piemontite, a rare-earth mineral, and about a dozen other minerals. All are monoclinic minerals that contain paired (SiO4) tetrahedra.
▪Zoisite Ca2Al3Si3O12(OH)
Origin of Name
Named after Baron von Zois (1747–1819), an Austrian who financed mineralogists.


Hand Specimen Identification
Occurrence in medium-grade metamorphic rocks is a key to identification. Zoisite often forms prismatic translucent or transparent crystals with vitreous or pearly lusters. Zoisite comes in a variety of colors and so can be confused with other minerals. The most common hues are white to gray, green, or green-brown. The crystals in these photos show examples from Baltistan, Pakistan.
Physical Properties
| hardness | 6 to 7 |
| specific gravity | 3.1 to 3.36 |
| cleavage/fracture | Perfect {010} imperfect {100}/uneven |
| luster/transparency | vitreous, pearly on cleavage surfaces/transparent to translucent |
| color | white, greenish gray, greenish brown, blue, purple, or pink |
| streak | white |
Properties in Thin Section
In thin section, zoisite has high relief, and is usually colorless and commonly shows dispersion. Interference colors are often anomalous; birefringence is low. Biaxial (+), α = 1.69, to 1.70 , β = 1.69 to 1.70, γ = 1.70 to 1.72, δ = 0.006 to 0.018, 2V = 0° to 70°.
Crystallography
Zoisite is orthorhombic, a = 8.94, b = 5.61, c = 10.23, Z = 2; space group $ \small{P \frac{2}{n} \frac{2}{m} \frac{2}{a}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Zoisite generally forms prismatic crystals that display striations. Columnar crystals , sometimes with terminating pyramids, are typical but it may also be massive
Structure and Composition
The zoisite structure is similar to epidote’s, containing both paired and unpaired (SiO4) tetrahedra, but it is overall orthorhombic.
Occurrences and Associations
Zoisite occurs in schists and other medium-grade metamorphic rocks and in pegmatites, less commonly, in eclogites or blueschists.
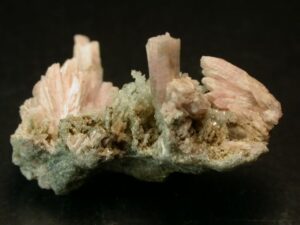

Varieties
Tanzanite is blue to purple gem quality zoisite (Figure 14.220). Thulite is a pink variety (Figure 14.221).
Related Minerals
Clinozoisite has a closely related structure but is monoclinic. Zoisite is structurally and chemically related to all the other paired tetrahedral silicates (see lawsonite related minerals).
▪Clinozoisite Ca2Al3Si3O12(OH)
Origin of Name
Named after zoisite, its orthorhombic polymorph.


Hand Specimen Identification
Clinozoisite is difficult to identify in hand specimen. It is most commonly green, like the specimens in these two photos, but various other colors are possible. When green, it may be mistaken for epidote; if uncolored or lightly colored, it is often overlooked. It cannot be distinguished with certainty from zoisite (orthorhombic) without X-ray analysis.
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 3.1 to 3.4 |
| cleavage/fracture | perfect (001)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | light to dark green, yellow, or gray |
| streak | white |
Properties in Thin Section
Clinozoisite has high relief, and is usually colorless. Interference colors are often anomalous; birefringence is low. Biaxial (+), α = 1.67, to 1.72 , β = 1.67 to 1.72, γ = 1.69 to 1.73, δ = 0.005 to 0.015, 2V = 14° to 90°
Crystallography
Clinozoisite is monoclinic, a = 8.94, b = 5.61, c = 10.23, β = 115.0°, Z = 2; space group $ \small{P \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Crystals of clinozoisite are prismatic, fibrous, or acicular, usually elongated parallel to the b-axis, with faces showing striations. Granular, massive, and fibrous aggregates are common.
Structure and Composition
The clinozoisite structure is similar to epidote’s, containing both paired and unpaired (SiO4) tetrahedra (see epidote). A complete solid solution exists between Fe-epidote, Ca2Fe3Si3O12(OH) and clinozoisite, Ca2Al3Si3O12(OH).
Occurrence and Associations
Clinozoisite, like epidote, is a product of metamorphism of Ca-rich rocks. It forms instead of epidote in relatively Fe-poor rocks.

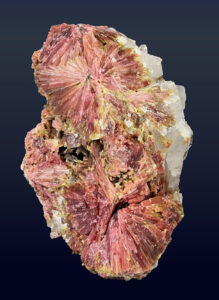

Varieties
Clinozoisite comes in many different colors besides green. Several examples are seen in these three photos. Pink varieties are called clinothulite.
Related Minerals
A polymorph of clinozoisite, named simply zoisite, is orthorhombic. Clinozoisite is structurally and chemically related to all the other paired tetrahedral silicates (see lawsonite related minerals).
▪Vesuvianite (Idocrase) Ca10(Mg,Fe)2Al4Si9O34(OH)4
Origin of Name
From the Italian Mount Vesuvius locality where this mineral was found.


Hand Specimen Identification
Occurrence in contact metamorphic rocks, translucent character, tetragonal or columnar habit, and brown or greenish color help identify vesuvianite. If not euhedral, identification can be problematic. It is sometimes confused with epidote, tourmaline, or grossular garnet. Figures 14.227 and 14.228 show examples.
Physical Properties
| hardness | 6.5 |
| specific gravity | 3.4 |
| cleavage/fracture | poor (001), (100), {110}/subconchoidal |
| luster/transparency | vitreous, resinous/transparent |
| color | brown, also yellow, green, blue, pink, or red |
| streak | white |
Properties in Thin Section
Vesuvianite is usually colorless but may be pleochroic in light green, brown, or yellow. High relief, low birefringence, and anomalous interference colors are typical. It may be confused with zoisite and clinozoisite but is uniaxial and lacks a good prismatic cleavage. Uniaxial (-), ω = 1.706, ε = 1.701, δ = 0.005.
Crystallography
Vesuvianite is tetragonal, a = 15.66, c = 11.85, Z = 4; space group $ \small{P \frac{4}{n} \frac{2}{n} \frac{2}{c}} $; point group $ \small{ \frac{4}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Typical vesuvianite occurs as coarse, prismatic, brown tetragonal prisms. Faces may be striated and crystals may be terminated by pyramids. Crystals may combine to form striated columnar masses, fibrous sheaves, or granular aggregates.
Structure and Composition
Vesuvianite, usually considered a paired tetrahedral silicate, contains both paired and unpaired (SiO4) tetrahedra. Its structural similarity to grossular leads to some misidentification. Composition is highly variable. Mn, Na, and K may replace Ca. Ti and Al may replace (Mg,Fe). Other elements, including B, Be, Cr, Cu, Li, Zn, and rare earths, may be present.
Occurrence and Associations
Vesuvianite is found primarily in contact aureoles associated with impure limestones or dolomites. Associated minerals include garnet, wollastonite, epidote, diopside, and carbonates. It is also found in altered mafic rocks, including serpentinites.

Varieties
Cyprine is a blue variety of vesuvianite (Figure 14.229)
Related Minerals
Vesuvianite is structurally and chemically similar to the garnet grossular; it is less similar to epidote and other paired tetrahedral silicates.
2 Native Elements
| Metals gold Au silver Ag platinum Pt copper Cu |
Semimetals arsenic As bismuth Bi antimony Sb |
Nonmetals diamond C graphite C sulfur S |
Gold, silver, platinum, and copper are the most common of the native metals. Additionally, iron, zinc, nickel, lead, and indium have occasionally been reported from meteorites or altered igneous rocks. All native metals have similar properties: metallic luster (if not tarnished), high thermal and electrical conductivity, malleability, and opaqueness to visible light. Complex solid solutions are possible, and many natural alloys have been given their own names. Kamacite and taenite, for example, are Fe-Ni alloys.
The native semimetals (arsenic, bismuth, and antimony), all rare, are found in hydrothermal deposits but rarely have economic importance. The native nonmetals are diverse in occurrence and properties. Graphite is common as an accessory mineral in many metamorphic rocks, sulfur exists in massive beds or as encrustations associated with fumaroles, and diamond is primarily restricted to kimberlite pipes, alluvium derived from kimberlites, or mantle nodules
For more general information about native elements, see the Section 9.2.1 in Chapter 9.
▪Gold Au
Origin of Name
The name of this mineral refers to its color.



Hand Specimen Identification
Gold is metallic and yellow. High specific gravity, sectile nature, and slightly different (more buttery yellow) color and luster distinguish gold from the yellow sulfides pyrite and chalcopyrite. Chalcopyrite, also, commonly tarnishes to obtain a slightly greenish hue, making it distinct from gold.
Physical Properties
| hardness | 2.5 to 3 |
| specific gravity | 15.6 to 19.3 |
| cleavage/fracture | none/hackly |
| luster/transparency | metallic/opaque |
| color | golden yellow |
| streak | gold-yellow |
Crystallography
Gold is cubic, a = 4.0783, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Gold crystals, when they exist, are octahedra, rarely showing other forms. More typically, gold is arborescent, fills fractures, or is found as nuggets, grains, or wire scales. Most gold crystals are exceptionally small.
Structure and Composition
Gold’s face-centered cubic structure is the same as the atomic arrangement in platinum and copper. Its composition is sometimes close to pure Au, but substantial Ag may be present in solid solution. Small amounts of other elements, such as Cu and Fe, may be present.
Occurrence and Associations
Gold is most often found in quartz veins associated with altered silicic igneous rocks. Associated minerals include quartz, pyrite, chalcopyrite, galena, stibnite, sphalerite, arsenopyrite, tourmaline, and molybdenite. It is also concentrated in placer deposits.
Varieties
Most natural gold contains up to 10% alloyed metals, thus giving rise to a number of slightly different colors and properties.
Related Minerals
Electrum is a name for intermediate Ag-Au solutions. Other gold-bearing minerals include calaverite (AuTe2), petzite (Ag3AuTe2), maldonite, (Au2Bi), and uytenbogaardtite (Ag3AuS3).
▪Silver Ag
Origin of Name
From the Old English word for this metal, seolfor.



Hand Specimen Identification
Silver may occur as cubic crystals, but more commonly has a wire-like, dendritic, or arborescent habit. It may have a metallic silver color, but only when fresh. Most of the time it tarnishes like the specimens seen in the three photos here. Silver has high specific gravity and is quite malleable. It is occasionally confused with the platinum group minerals. If cubic and tarnished it may be confused with galena.
Physical Properties
| hardness | 2.5 to 3 |
| specific gravity | 10.1 to 10.5 |
| cleavage/fracture | none/hackly |
| luster/transparency | metallic/opaque |
| color | silver-white but typically tarnished |
| streak | silver-white |
Crystallography
Silver is cubic, a = 4.0856, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Distorted cubes, octahedra, or dodecahedra are known, but silver is typically acicular. Flakes, plates, scales, and filiform or arborescent masses are common.
Structure and Composition
Silver has a face-centered cubic structure that is isostructural with copper. It may contain substantial amount of Au, Hg, Cu, As, Sb, Bi, Pt, or Fe in solid solution.
Occurrence and Associations
Silver is found with sulfides and arsenide in oxidized zones of ore deposits, or in hydrothermal deposits. The many associated minerals include, most significantly, species containing Co, Ni, and As.
Varieties
Amalgam is a solid solution of Ag and Hg. Electrum is a solid solution of Ag and Au.
Related Minerals
Silver is isostructural with copper. Other Ag minerals include dyscrasite (Ag3Sb), argentite (Ag2S), proustite (Ag3AsS3), and pyrargyrite (Ag3Sb3).
▪Platinum Pt
Origin of Name
From the Spanish platina, meaning “silver.”

Hand Specimen Identification
Platinum is most easily identified by its malleability, silvery-gray color and streak, and very high specific gravity. Although a cubic mineral, euhedral crystals (generally distorted cubes) are rare; it most commonly occurs as nuggets, often with rounded corners, like the nuggets seen in top row of Figure 14.236.
Physical Properties
| hardness | 4 to 4.5 |
| specific gravity | 21.47 |
| cleavage/fracture | none/hackly |
| luster/transparency | metallic/opaque |
| color | gray-silver, steel-gray |
| streak | gray-silver, steel-gray |
Crystallography
Platinum is cubic, a = 3.9237, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Euhedral or subhedral platinum crystals, generally poorly formed, are exceptional. Masses, nuggets, or small grains are typical.
Structure and Composition
Platinum has a cubic closest packed structure similar to gold’s structure. It forms alloys with other elements, notably Fe, Cu, Pd, Rh, and Ir.
Occurrence and Associations
Primary platinum is found with chromite, spinel, and olivine in ultramafic rocks. It is also found in some placer deposits.
Related Minerals
Platinum is isotypical with copper.
▪Copper Cu
Origin of Name
From the Greek word kyprios, referring to Cyprus, one of the earliest places where copper was mined.



Hand Specimen Identification
Native copper has a copper-red or pale rose-red color, and most commonly forms as scales of flakes (Figures 14.237 and 14.238), or branching arborescent crystals (Figure 14.239). It commonly tarnishes, has a hackly fracture, is malleability, and has high specific gravity. The photos in Figures 14.237 and 14.239 show copper that is altering to green malachite (Cu-carbonate).
Physical Properties
| hardness | 2.5 to 3 |
| specific gravity | 8.7 to 8.9 |
| cleavage/fracture | none/hackly |
| luster/transparency | metallic/opaque |
| color | copper color, copper-red or rose-red; sometimes tarnished |
| streak | copper-red |
Crystallography
Copper is cubic, a = 3.6153, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Copper may form cubes, octahedra, or dodecahedra. When euhedral, contact or penetration twins are common. Most copper is in the form of malformed crystals, or dendritic, arborescent, or irregular plates, scales, or masses.
Structure and Composition
Copper has a cubic closest packed structure similar to gold and platinum. It often contains solid solutions of Ag, Fe, As, or other elements.
Occurrence and Associations
Copper is found in the oxidized zones of many copper deposits, and as primary mineralization from hydrothermal fluids passing through mafic lavas. Copper is often deposited in voids or cracks. Associated minerals include silver, sulfides, calcite, chlorite, zeolites, cuprite, malachite, and azurite.
Related Minerals
Gold, silver, platinum, and lead are isotypical with copper.
▪Diamond C
Origin of Name
From the Greek word adamas, meaning “invincible.”


Hand Specimen Identification
Diamond is distinguished by its occurrences, hardness, octahedral cleavage and sometimes octahedral shape, and luster. Diamonds are mined from alluvial (placer) deposits and from kimberlite pipes. Figure 14.240 shows alluvial diamonds, and Figure 14.241 shows a large diamond in kimberlite.
Physical Properties
| hardness | 10 |
| specific gravity | 3.5 |
| cleavage/fracture | perfect octahedral {111}/conchoidal |
| luster/transparency | adamantine/transparent |
| color | typically colorless but rare, colored varieties may be valuable |
| streak | white |
Properties in Thin Section
Diamond is isotropic, n = 2.419.
Crystallography
Diamond is cubic, a = 3.5668, Z = 8; space group $ \small {F \frac{4}{d} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Diamond crystals are usually octahedral and often distorted or twinned. More rarely, diamond forms cubes or dodecahedra. Curved faces are common.
Structure and Composition
Diamond is essentially pure carbon but may contain inclusions of other material.
Occurrence and Associations
Diamond is found in altered ultramafic rock of mantle origin or in placer deposits. Associated minerals include pyrope, olivine, kyanite, and zircon.
Related Minerals
Graphite is a polymorph of diamond.
▪Graphite C
Origin of Name
The name comes from the Greek word graphein, meaning “to write,” because of its use in pencils.


Hand Specimen Identification
Graphite is easily recognized by its greasy feel, softness, shiny luster, dark color and streak, and foliated nature. Figure 14.242 shows a typical massive example. Occasionally graphite is in the form of hexagonal crystals but they are generally quite small. Figure 14.243 shows two examples.
Physical Properties
| hardness | 1 to 2 |
| specific gravity | 2.1 to 2.2 |
| cleavage/fracture | perfect basal (001)/elastic, flexible |
| luster/transparency | submetallic/opaque |
| color | lead-gray, black |
| streak | black |
Crystallography
Graphite is hexagonal, a = 2.46, c = 10.06, Z = 6; space group $ \small {R\overline{3}\frac{2}{m}}$; point group $ \small {\overline{3}\frac{2}{m}}$.
Habit
Well-formed graphite crystals are hexagonal tablets. Foliated and scaly masses are common; radiating or granular aggregates are less common.
Structure and Composition
Graphite‘s structure contains stacked planes of covalently bonded C atoms arranged in a hexagonal pattern. Graphite is essentially pure carbon.
Occurrence and Associations
Graphite is common in a wide variety of metamorphic rocks including schists, marbles, and gneisses. It is a rare mineral in some igneous rocks. Graphite is usually disseminated as fine flakes, but may form large books.
Related Minerals
Graphite is a polymorph of graphite.
▪Sulfur S
Origin of Name
From the Middle English word sulphur, meaning “brimstone.”


Hand Specimen Identification
Sulfur can be easily identified by its yellow color, hardness, density, and sometimes eggy odor. It is occasionally confused with orpiment, the only other relatively common yellow mineral, or yellow sphalerite.
Physical Properties
| hardness | 2 |
| specific gravity | 2.1 |
| cleavage/fracture | poor {101} and {110}/ conchoidal |
| luster/transparency | resinous or dull/transparent to translucent |
| color | bright yellow |
| streak | white |
Properties in Thin Section
Sulfur is characterized by extreme relief and birefringence. It is pale yellow in thin section and commonly pleochroic. Biaxial, α = 1.958, β = 2.038, γ = 2.245, δ = 0.29, 2V = 69o.
Crystallography
Sulfur crystals are orthorhombic, a = 10.44, b = 12.84, c = 24.37, Z = 128; space group $ \small{F \frac{2}{d} \frac{2}{d} \frac{2}{d}} $; point group $ \small{\frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Typically, sulfur is massive, colloform, or stalactitic, but tabular crystals may display combinations of orthorhombic prisms, dipyramids and pinacoids.
Structure and Composition
The sulfur structure consists of covalently bonded groups, stacked parallel to the c-axis, and weakly connected to each other. Sulfur is essentially pure S but may contain small amounts of Se in solid solution.

Occurrence and Associations
Sulfur is found as a deposit associated with volcanic fumaroles (Figure 14.246). It also occurs in veins where it forms from sulfides, or in sediments where it forms by the reduction of sulfates by bacterial action. The most substantial occurrences are thick evaporite beds in sedimentary sequences. Associated minerals include other sulfur-containing minerals (celestite, gypsum, anhydrite), and carbonates.
Related Minerals
Sulfur has several different polymorphs.
3 Sulfide Minerals
Sulfides and related minerals vary greatly in atomic arrangement. In this book, we divide them into tetrahedral sulfides, octahedral sulfides, and other sulfides. In tetrahedral sulfides, all metal atoms are in 4-fold coordination. In octahedral sulfides, all metal atoms are in 6-fold coordination. And, in the third category, metal coordination is unusual, for example 5-fold coordination, or mixed. Bonding is generally some combination of ionic and covalent, but some sulfides have significant metallic character.
Many sulfides share common properties, sometimes making distinguishing them problematic. For example, common metallic sulfides include pyrite, chalcopyrite, molybdenite, galena, acanthite, chalcocite, bornite, pyrrhotite, millerite, pentlandite, stibnite, marcasite, cobaltite, arsenopyrite, tetrahedrite, argentite, and niccolite. Of these, pyrite, chalcopyrite, pyrrhotite, millerite, and pentlandinte have brass to yellow to gold colors and are occasionally confused. Molybdenite, galena, acanthite, chalcocite, stibnite, tetrahedrite, and enargite have gray to black colors and so, too, are sometimes difficult to distinguish.
Sulfide minerals have variable compositions and a wide variety of complex solid solutions are possible, especially at high temperature. At low temperature, however, many sulfide minerals exhibit exsolution resulting from unmixing to form more stable phases.
In this book, we group the sulfide and (the much less common) arsenide minerals together because they share many properties. In these minerals, sulfur and arsenic are nearly closest packed with metals between. The simplest sulfide minerals, such as galena (PbS), have atomic arrangements similar to the arrangement in halite – symmetrical arrangements of metal atoms alternating with S, resulting in cubic or hexagonal crystals.
3.1 Tetrahedral Sulfides and Arsenides
| Tetrahedral Sulfide Group Minerals sphalerite ZnS wurtzite ZnS chalcopyrite CuFeS2 bornite Cu5FeS4 enargite Cu3AsS4 |
In some sulfides and arsenides, all metal atoms are in tetrahedral coordination. Five examples are listed in the table here and described in more detail below.
Fore more general information about sulfide minerals, see the discussion in Section 9.2.2, Chapter 9.
▪Sphalerite ZnS
Origin of Name
This mineral’s name comes from the Greek word sphaleros, meaning “treacherous,” alluding to problems identifying the mineral.



Hand Specimen Identification
Sphalerite is the most important zinc ore mineral. It has many different appearances. It is a dense mineral with perfect cleavage but comes in many colors. It is most easily recognized when it has a brown to yellow resinous to adamantine luster (Figure 14.247). But sphalerite may be black, it may have a metallic luster (Figure 14.248), and it may occur as light brown or yellow clear to translucent crystals (Figure 14.249).
Sphalerite is sometimes confused with galena, siderite, sulfur, or enargite. If it has a dark color, a brownish streak may help distinguish it from other dark-colored minerals (which generally have a gray or black streak).
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 4.0 |
| cleavage/fracture | perfect dodecahedral {110}/conchoidal |
| luster/transparency | highly variable, adamantine to resinous, sometimes metallic/ transparent to opaque |
| color | variable; colorless when pure, otherwise often shades of brown or shades of orange, red, yellow, and sometimes black |
| streak | white to brown or yellow |
Properties in Thin Section
Sphalerite is colorless to pale brown or yellow in thin section. It has extremely high relief and good cleavage. Isotropic, n = 2.42.
Crystallography
Sphalerite is a cubic mineral, a = 5.785, Z = 4. space group $ \small {F \overline{4}3m} $; point group $ \small {\overline{4}3m} $.
Habit
Sphalerite crystals may be distorted or rounded. Crystals show combinations of tetrahedra, dodecahedra, and cubes. Polysynthetic twinning is common. Sphalerite commonly forms cleavable masses. It is typically brown and resinous but has many other guises.
Structure and Composition
Sphalerite is isostructural with diamond, with S arranged in a face-centered cubic pattern and Zn occupying tetrahedral sites within the cube. Many solid solutions are possible. Fe and, to a lesser extent, some Mn and Cd are nearly always present.
Occurrence and Associations
Sphalerite is a common mineral found in several different kinds of deposits. It is found with galena, chalcopyrite, pyrite, barite, fluorite, carbonates, and quartz in voids and fracture fillings of carbonate hosts. It is found in hydrothermal veins with pyrrhotite, pyrite, and magnetite, and is also found in contact metamorphic aureoles.

Varieties
Orange to yellow sphalerite is called golden sphalerite, red sphalerite is sometimes called ruby blende or ruby jack. High-Fe sphalerite may be a black submetallic variety called marmatite (Figure 14.250).
Related Minerals
Wurtzite is a high-temperature hexagonal polymorph of sphalerite. Greenockite, CdS, is isostructural with sphalerite at low temperature and with wurtzite at high temperature.
▪Chalcopyrite CuFeS2
Origin of Name
From the Greek word chalkos, meaning “copper,” and pyrites, meaning “to ignite.”



Hand Specimen Identification
Metallic luster, a brass-yellow color, and a greenish-black streak help identify chalcopyrite. Figure 14.251 is a photo of chalcopyrite on sphalerite. Chalcopyrite typically tarnishes and may become greenish (Figure 14.252) or other colors (Figure 14.253). It may be confused with pyrite, but has a more yellow color, is softer, and pyrite does not tarnish.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 4.2 |
| cleavage/fracture | poor {011}/uneven |
| luster/transparency | metallic/opaque |
| color | brass-yellow, sometimes tarnished showing green or orange hues |
| streak | greenish black |
Crystallography
Chalcopyrite crystals are tetragonal, a = 5.25, c = 10.32, Z = 4; space group $ \small {I \overline{4}2d} $; point group $ \small {\overline{4}2m}$.
Habit
Chalcopyrite crystals are usually pseudotetrahedral, displaying disphenoidal faces, sometimes in combination with prisms. Polysynthetic and penetration twins are common. Massive aggregates are common.
Structure and Composition
Chalcopyrite‘s structure is similar to that of sphalerite. Cu and Fe alternate in tetrahedral sites between S arranged in a face-centered cubic pattern. Small amounts of Ag, Au, Zn, and other elements are commonly present.
Occurrence and Associations
Chalcopyrite, the most important Cu ore mineral, is widespread and common. It is present in most sulfide deposits, but the most significant ores are formed by hydrothermal veins or by replacement. Common associated minerals include pyrite, sphalerite, bornite, galena, and chalcocite. Chalcopyrite is also found as magmatic segregations associated with pyrrhotite and pentlandite and in black shales. Alteration of chalcopyrite leads to association with malachite, azurite and other less common secondary copper minerals.
Related Minerals
A number of other sulfides are isotypical with chalcopyrite, including stannite, Cu2FeSnS4; gallite, CuGaS2; and roquesite, CuInS2. Other Cu-Fe minerals include talnakhite, Cu9(Fe,Ni)8S16; mooihoekite, Cu9Fe9S16; and haycockite, Cu4Fe5S8.
▪Bornite Cu5FeS
Origin of Name
Named after Ignaz von Born (1742–1791), a German mineralogist.
Hand Specimen Identification
When untarnished, bornite has a copper-red to bronzish-brown color (Figure 14.254), sometimes with a greenish hue. However, this mineral typically tarnishes to iridescent shades of blue, purple, red or other colors (Figures 14.255 and 14.256). Its colorful metallic luster, and common iridescence are keys to identification. Purplish blue and violet varicolored specimens, such as the one seen in Figure 14.257, are called peacock ore. Bornite may occasionally be confused with niccolite, pyrrhotite, chalcocite, and covellite.
Physical Properties
| hardness | 3 |
| specific gravity | 6 |
| cleavage/fracture | poor {111}/conchoidal |
| luster/transparency | metallic/opaque |
| color | copper-red to bronzish-brown if not tarnished; more typically iridescent purple or blue |
| streak | grayish black |
Crystallography
Bornite is tetragonal, a = 10.94, c = 21.88, Z = 16; space group $ \small {I \overline{4}2d} $; point group $ \small {\overline{4}2m}$.
Habit
Bornite crystals are often pseudocubic, less commonly pseudododecahedral and pseudooctahedral. Tetragonal crystals with distorted or curved faces are rarer. Massive aggregates are common.
Structure and Composition
Bornite‘s structure is complex. S is distributed in a modified face-centered cubic arrangement. Cu and Fe are in tetrahedral sites, each coordinated to four S. The structure typically contains many defects. At high temperatures bornite forms solid solutions with chalcopyrite, CuFeS2. Consequently, Cu:Fe ratios are somewhat variable. If cooling is slow, exsolution occurs. Small amounts of Pb, Au, Ag, and other elements may also be present.
Occurrence and Associations
The most important bornite occurrences are in sulfide veins and as a secondary mineral in enriched zones of sulfide deposits. Typical associated minerals include chalcopyrite, chalcocite, covellite, pyrrhotite, pyrite, and quartz.
Related Minerals
A cubic polymorph of bornite exists above 228°C (440° F). Related minerals include chalcopyrite, CuFeS2, and pentlandite, (Ni,Fe)9S8.
▪Enargite Cu3AsS4
Origin of Name
From the Greek word enarges, meaning “distinct,” referring to its cleavage.


Hand Specimen Identification
Enargite is sometimes difficult to distinguish from other dense dark-colored minerals such as stibnite or sphalerite. Its key properties include high specific gravity (4.5), softness (H = 3), prismatic cleavage, and dark gray to black color. These two photos show typical examples.
Physical Properties
| hardness | 3 |
| specific gravity | 4.5 |
| cleavage/fracture | perfect prismatic {110}, good (100), good (010), poor (001)/uneven |
| luster/transparency | metallic/opaque |
| color | violet black, grayish black, iron black |
| streak | dark gray, gray-black |
Crystallography
Enargite is orthorhombic, a = 6.47, b = 7.44, c = 6.19, Z = 1; space group $ \small{Pnm2} $; point group $ \small{ mm2} $.
Habit
Enargite crystals are tabular or columnar, and striated. Massive, columnar, or granular aggregates are common.
Structure and Composition
Enargite‘s closest packed structure is closely related to that of wurtzite, the hexagonal polymorph of sphalerite. Sb commonly substitutes for some As in enargite. Some Fe, Zn, and Ge replace Cu.
Occurrence and Associations
Enargite is found in vein or replacement sulfide deposits with other sulfides such as chalcocite, covellite, galena, bornite, sphalerite, and pyrite.
Related Minerals
Enargite is the most common of the sulfosalts, a group of minerals similar to sulfides, but that have S, As, Sb, or Bi in chains or sheets. Other related sulfosalts are pyrargyrite, Ag3SbS3; tetrahedrite, Cu12Sb4S13; and tennantite, Cu12As4S13. Enargite has a rare very low temperature tetragonal polymorph, luzonite. Luzonite is isostructural with famatinite, Cu3SbS4, with which enargite forms a partial solid solution. Other related minerals are sulvanite, Cu3VS4, and germantite, Cu3GeS4.
3.2 Octahedral Sulfide Group Minerals
| Octahedral Sulfide Group Minerals galena PbS pyrrhotite Fe1-xS niccolite NiAs |
Galena and pyrrhotite are the two most important octahedral sulfides; niccolite, although not containing sulfur, is included in this group because of structural similarities. In the octahedral sulfide structure, S and As are closest packed and metal atoms occupy only octahedral sites.
Fore more general information about sulfide minerals, see the discussion in Chapter 9.
▪Galena PbS
Origin of Name
From the Latin word galene, a name originally given to lead ore.



Hand Specimen Identification
Galena is recognized by its density, silver-gray metallic appearance, blocky (often cubic) habit, good cleavage, and softness. Figure 14.260 shows a typical galena cube.
Galena commonly alters to secondary minerals. Figure 14.261 is a photo of subhedral galena accompanied by clear tan and yellow anglesite (PbSO4). Galena does not always for cubes, the crystals in Figure 14.262 are not cubes but, instead, are pseudododecahedral. See also the photo of galena with pyrrhotite in Figure 14.264, below.
Physical Properties
| hardness | 2.5 |
| specific gravity | 7.6 |
| cleavage/fracture | perfect {100}/subconchoidal |
| luster/transparency | metallic/opaque |
| color | lead-gray |
| streak | lead-gray |
Crystallography
Galena is a cubic mineral, a = 5.94, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Galena crystals are typically cubes or cubes modified by octahedra; less commonly they contain other cubic forms. Penetration and contact twins are common. Lamellar twins are less common. Aggregates of galena crystals are massive, finely granular, or plumose.
Structure and Composition
The structure of galena is similar to that of halite. Alternating Pb and S are arranged in a face-centered cubic pattern. Galena often contains small amounts of Fe, As, or Sb, and even smaller amounts of Zn, Cd, Bi, and Se. Other impurities may be present in trace amounts.
Occurrence and Associations
Galena is common in many types of sulfide deposits. Associated minerals include sphalerite, chalcopyrite, pyrite, fluorite, barite, marcasite, cerussite, anglesite, calcite, dolomite, and quartz. Galena is often associated with silver minerals such as silver, acanthite, or pyrargyrite.
Related Minerals
Besides halite (NaCl), other minerals isostructural with galena include periclase (MgO), wüstite (FeO), alabandite (MnS), altaite (PbTe), and clausthalite (PbSe).
▪Pyrrhotite Fe1-xS
Origin of Name
From the Greek word pyrrhos, meaning “flame colored.”


Hand Specimen Identification
Pyrrhotite is recognized by its metallic luster, and its (usual) gold or bronze color that tarnishes to a reddish-brown hue. Tarnishing sometimes produces a faint blue to red iridescence. Pyrrhotite is also weakly magnetic. It may be easily mistaken for pyrite, pentlandite, or bornite – other typically gold metallic minerals – unless it is tarnished.
Figure 14.263 shows a golden “barrel” of untarnished pyrrhotite from Russia. It appears quite similar to pyrite. Figure 14.264 shows tarnished pyrrhotite from Mexico. The tarnishing identifies this mineral. The specimen also includes gray galena.
Physical Properties
| hardness | 4 |
| specific gravity | 4.6 |
| cleavage/fracture | poor (001)/uneven |
| luster/transparency | metallic/opaque |
| color | golden yellow to reddish bronze |
| streak | gray to black |
Crystallography
Pyrrhotite is a monoclinic mineral but has a hexagonal polytype. a = 11.88, b = 6.87, c = 22.79, β = 90.47 Z = 26; space group $ \small{A\frac{2}{a}} $; point group $ \small{\frac{2}{m}} $.
Habit
Pyrrhotite may be massive or disseminated. Rare crystals are hexagonal plates or tabs, often twinned.
Structure and Composition
Pyrrhotite‘s complex structure is similar to the structure of niccolite (NiAs). Fe occupies sites between hexagonally closest packed S. The amount and distribution of Fe are complex functions of composition and crystallization history, so composition is variable. Most pyrrhotite has less Fe than S. Ni, Co, Mn, and Cu are often present in small amounts.
Occurrence and Associations
Pyrrhotite is typically found in mafic igneous rocks. Associated minerals include pyrite, pentlandite, galena, magnetite, and chalcopyrite. Other pyrrhotite occurrences are in pegmatites, contact aureoles, and vein deposits.
Related Minerals
Pyrrhotite has a hexagonal polymorph stable at high temperature. Pyrrhotite is generally slightly deficient in Fe, which is why its formula is written as Fe1-xS. Troilite is end-member FeS. Minerals that are isotypical with pyrrhotite include troilite (FeS), niccolite (NiAs), and breithauptite (NiSb).
▪Niccolite NiAs
Origin of Name
The name refers to this mineral’s nickel content.



Hand Specimen Identification
Niccolite is often easily recognized by its pale metallic copper-red color. Figure 14.265 shows a good example of reddish niccolite surrounded by quartz. The niccolite in Figure 14.266, on top of barite, is not quite as red-colored.
Alteration to a green hydrated nickel arsenate called nickel bloom, or annabergite, is diagnostic for this mineral. Figure 14.267 is a photo of dark-colored tarnished niccolite with green annabergite. Some annabergite has a much brighter emerald-green color than the green seen in this photo.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 4.6 |
| cleavage/fracture | poor (001)/uneven |
| luster/transparency | metallic/opaque |
| color | copper-red |
| streak | brownish black |
Crystallography
Niccolite is hexagonal, a = 3.58, c = 5.11, Z = 2; space group $ \small{P \frac{6_3}{m} \frac{2}{m} \frac{2}{c}} $; point group $ \small{\frac{6}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Rare crystals are tabular with pyramidal faces and sometimes cyclic twins. Niccolite is usually massive and sometimes colloform or columnar.
Structure and Composition
Niccolite‘s structure involves hexagonal closest packed As with Ni between. Sb usually replaces some of the As; Fe, Co, and S are also present in small amounts.
Occurrence and Associations
Niccolite is found in veins with Co and Ag minerals and in sulfide deposits hosted by mafic igneous rocks. Associated minerals include pyrrhotite, chalcopyrite, skutterudite, silver, and a variety of other sulfosalts.
Related Minerals
Breithauptite, NiSb; freboldite, CoSe; and kotulskite, Pd(Te,Bi) are isostructural with niccolite. Other related minerals include millerite, NiS; pentlandite, (Ni,Fe)9S8; and langisite, (Co,Ni)As. Annabergite, Ni3As2O8•8H2O, also called nickel bloom, is a common green alteration product of niccolite.
3.3 Other Sulfide Minerals
| Other Sulfides | |
| pentlandite (Ni,Fe)9S8 molybdenite MoS2 millerite NiS cinnabar HgS covellite CuS chalcocite Cu2S argentite (acanthite) Ag2S pyrite FeS2 cobaltite (Co,Fe)AsS |
marcasite FeS2 arsenopyrite FeAsS skutterudite (Co,Ni)As3 stibnite Sb2S3 tetrahedrite Cu12Sb4S13 pyrargyrite (ruby silver) Ag3SbS3 orpiment As2S3 realgar AsS |
The eight sulfides just discussed and several other less important ones have relatively simple structures based on closest packing and metal ions occupying either tetrahedral or octahedral sites, but not both. Pentlandite, the most important ore mineral of nickel, is the only common sulfide containing metal atoms in both coordinations. Many other sulfides, including those listed in the blue box, have more complex structures. Metal ions may be in 5-fold or other unusual coordinations, they may occupy several different sites in the structures, and the sites may be highly polarized or distorted. Below we look at some of these other sulfides.
For more general information about sulfide minerals, see the discussion in Chapter 9.
▪Pentlandite (Ni,Fe)9S8
Origin of Name
Named after J. B. Pentland (d. 1873), a geologist working in Sudbury, Ontario, Canada, who first described the mineral.

Hand Specimen Identification
Metallic luster, bronze-yellow color and association help identify pentlandite, but distinguishing it from other golden or brassy minerals is problematic without chemical analysis. It resembles pyrrhotite in appearance but is not magnetic. Often the two minerals are found together; Figure 14.268 shows an example from Norway. In this specimen, pentlandite has a shiny metallic gold/brass color. The pyrrhotite is tarnished and has a much duller luster.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 5.0 |
| cleavage/fracture | perfect {100}, good octahedral {111}/uneven |
| luster/transparency | metallic/opaque |
| color | bronze to yellow-bronze |
| streak | light bronze to brown |
Crystallography
Pentlandite is cubic, a = 10.05, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Coarse crystals are very rare. Pentlandite is usually massive or in granular aggregates. Sometimes {111} parting develops.
Structure and Composition
Pentlandite has a complicated face-centered cubic structure. The basic structure consists of (Ni,Fe)S6 octahedra sharing corners. Additional Ni and Fe occupy distorted tetrahedral sites between the octahedra. Co commonly substitutes for (Ni,Fe). Mn and Cu are other common impurities.
Occurrence and Associations
Pentlandite, the most important nickel ore mineral, is found in late-stage sulfide deposits with other nickel minerals (millerite, niccolite), pyrrhotite, and chalcopyrite. Pentlandite often occurs as exsolved blebs and lamellae within pyrrhotite.
Related Minerals
Pentlandite forms solid solutions with cobalt pentlandite, Co9S8. It is isostructural with a number of minerals, including argentopentlandite, Ag(Fe,Ni)8S8. Other related minerals are bornite, Cu5FeS, and niccolite, NiAs.
▪Molybdenite MoS2
Origin of Name
From the Greek word molybdos, meaning “lead,” which refers to a misidentification by early mineralogists.


Hand Specimen Identification
Metallic luster, silver/gray color, softness, flexibility, and basal cleavage identify molybdenite. It sometimes resembles graphite, another mineral that may occur as silver/gray metallic hexagonal crystals. Figure 14.269 shows a hexagonal flake of molybdenite in quartz.
Molybdenite, like the flake in the photo above, often appears more metallic than graphite. Additionally, graphite‘s color and streak are black to gray; molybdenite‘s color and streak may be more bluish gray. Molydenite is often anhedral and massive; the specimen seen in Figure 14.270 is an example. Figure 9.35 is a photo of another example of massive molybdenite.
Physical Properties
| hardness | 1 to 1.5 |
| specific gravity | 4.7 |
| cleavage/fracture | perfect basal (001)/flexible |
| luster/transparency | metallic/opaque |
| color | silver, lead-gray, sometimes with a hint of blue |
| streak | gray or blue-gray |
Crystallography
Molybdenite is hexagonal, a = 3.16, c = 12.32, Z = 2; space group $ \small{P \frac{6_3}{m} \frac{2}{m} \frac{2}{m}} $; point group $ \small{\frac{6}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Molybdenite crystals form hexagonal plates or stubby prisms. Foliated or scaly aggregates are flexible, but not elastic.
Structure and Composition
The molybdenite structure involves two sheets of S, arranged in a hexagonal pattern, sandwiching a sheet of Mo atoms. Each Mo atom is bonded to three S in each of the two sheets. The three-layer units are stacked up to produce the entire structure. Molybdenite is usually quite close to its stoichiometric composition but may contain traces of Au, Ag, Re, and Se.
Occurrence and Associations
Molybdenite occurs as an accessory mineral in some granitic rocks, including pegmatites. It also is found in porphyry copper deposits; in vein deposits with scheelite, cassiterite, wolframite, and fluorite; and in some contact aureoles.
▪Millerite NiS
Origin of Name
Named after W. H. Miller (1801–1880), who was the first to study the crystals.


Hand Specimen Identification
Millerite is easily recognized if it forms radiating acicular crystals, and it often does (Figure 14.271). The specimen seen in Figure 14.272 also contains needles of millerite but they are coarser and do not appear to radiate. Luster, brass-yellow color (if present), and rhombohedral cleavage also aid identification. Otherwise, identification may be problematic.
Physical Properties
| hardness | 3 to 3.5 |
| specific gravity | 5.5 |
| cleavage/fracture | perfect {101} and {012}/uneven |
| luster/transparency | metallic/opaque |
| color | bronze, greenish gray, gray, or brass yellow |
| streak | greenish black, green-gray |
Crystallography
Millerite forms trigonal crystals. a = 9.62, c = 3.15, Z = 9; space group $ \small{R3m}$; point group $ \small{3m}$.
Habit
Crystals are typically acicular or filiform. Millerite may form radiating sprays or velvety crusts.
Structure and Composition
The structure of millerite is a complex derivative of the niccolite structure. Both Ni and S are in 5-fold coordination. Co, Fe, and As are minor impurities.
Occurrence and Associations
Millerite is a low-temperature mineral that forms as a replacement for other nickel minerals or in cavities. It is associated with calcite, fluorite, dolomite, hematite, siderite, pyrrhotite, and chalcopyrite.
Related Minerals
Millerite has structural similarity with niccolite, NiAs, and with pyrrhotite, Fe1-xS. A high-temperature polymorph exists above 379 °C (714 °F).
▪Cinnabar HgS
Origin of Name
The origin of the name is uncertain.


Hand Specimen Identification
High density, red color, and streak identify cinnabar, the most common mercury mineral. It may be confused with hematite, cuprite, or realgar, mostly due to its red color.
Figure 14.273 show euhedral cinnabar crystals on top of dolomite. Most cinnabar, however, is massive like the cinnabar seen in Figure 14.274. The photo also contain blebs of silvery native mercury (a liquid). The specimen comes from a famous mercury mine district in Almaden, Spain.
Physical Properties
| hardness | 2.5 |
| specific gravity | 8.1 |
| cleavage/fracture | perfect prismatic {100}/ subconchoidal |
| luster/transparency | adamantine/transparent to translucent |
| color | bright red to brownish red |
| streak | scarlet |
Properties in Thin Section
Cinnabar is uniaxial (+), ω = 2.90, ε = 3.25, δ = 0.35.
Crystallography
Cinnabar crystals are trigonal. a = 4.15, c = 9.50, Z = 3; space group $ \small{P312} $; point group $ \small{32}$.
Habit
Rare cinnabar crystals are rhombohedral, thick tabs or prisms; less commonly acicular. Most occurrences are granular or earthy masses; often they are crusty, sometimes disseminated.
Structure and Composition
Cinnabar’s structure consists of Hg-S-Hg chains spiraling parallel to the c-axis. It is usually close to HgS in composition; only traces of other elements are present.
Occurrence and Associations
Cinnabar is the most significant Hg ore mineral. It is found as masses in volcanic or sedimentary rocks, in veins, or as disseminated grains. Associated minerals include native mercury, realgar, stibnite, pyrite, marcasite, calcite, quartz, and opal.
Related Minerals
Metacinnabar (cubic) and hypercinnabar (hexagonal) are polymorphs of cinnabar. Other related mercury minerals include coloradoite (HgTe), and tiemannite (HgSe).
▪Covellite CuS
Origin of Name
Named after N. I. Covelli (1790–1829), who discovered Vesuvian covellite.


Hand Specimen Identification
High density, shiny luster, distinctive indigo-blue color, and association identify covellite. The photos seen here show two examples of covellite from Butte, Montana, where significant amounts of copper were produced from the late 1800s to around 1983. Euhedral crystals, such as those seen in Figure 14.275 are rare but spectacular. Figure 9.38 shows another example from Butte.
Physical Properties
| hardness | 1.5 to 2 |
| specific gravity | 4.6 |
| cleavage/fracture | perfect basal (001)/conchoidal |
| luster/transparency | metallic/opaque |
| color | indigo-blue; purplish tarnish |
| streak | dark gray to black |
Crystallography
Covellite crystals are hexagonal, a = 3.80, c = 16.36, Z = 6; space group $ \small{P \frac{6_3}{m} \frac{2}{m} \frac{2}{c}} $; point group $ \small{\frac{6}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Rare hexagonal crystals are tabular or platy; covellite is usually in massive or foliated aggregates or in overgrowths and coatings on other copper minerals.
Structure and Composition
In covellite, covalent sulfur bonds link layers of CuS4 tetrahedra. Weak bonds between the layers result in excellent planar cleavage. Fe often replaces some Cu; Se replaces some S.
Occurrence and Associations
Primarily a secondary (supergene) mineral, covellite occurs with other Cu sulfides in veins or disseminated deposits. Associated minerals include bornite, chalcopyrite, chalcocite, and enargite.
Related Minerals
Covellite is similar in some ways to klockmannite (CuSe), with which it forms solid solutions.
▪Chalcocite Cu2S
Origin of Name
From the Greek word chalkos, meaning “copper.”


Hand Specimen Identification
Gray, often sooty color with a blue tarnish, luster, hardness, sectile nature, and density may identify chalcocite. Association with other copper minerals is helpful, but otherwise chalcocite can be confused with other gray nondescript minerals.
The photo on the left (Figure 14.277) shows a sample of massive chalcocite from Bisbee, Arizona, where copper was mined from about 1875 to 1975. The photo on the right (Figure 14.278) contains a mass of euhedral chalcocite crystals from Queensland, Australia.
Physical Properties
| hardness | 2.5-3 |
| specific gravity | 5.8 |
| cleavage/fracture | poor prismatic {110}/ conchoidal |
| luster/transparency | metallic/opaque |
| color | blue-white, shining lead-gray, dull sooty gray |
| streak | grayish black |
Crystallography
Chalcocite is monoclinic, a = 15.25, b = 11.88, c = 13.49, β = 116.35°, Z = 48; space group $ \small{P \frac{2_1}{c}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Chalcocite is usually fine grained and massive with conchoidal fracture. Squat prisms or tabular
crystals, sometimes with a hexagonal outline, and sometimes displaying striations, are rare.
Structure and Composition
Chalcocite‘s structure is based on hexagonal close packed sulfur atoms. Two-thirds of the Cu atoms occupy trigonal sites within sulfur planes; the other third is in octahedral coordination between planes. Fe and Ag are common replacements for Cu; Se may replace some S.
Occurrence and Associations
Chalcocite is a common primary or secondary copper ore mineral. It occurs both in veins and in altered zones. Associated primary minerals include bornite, chalcopyrite, enargite, galena, tetrahedrite, cuprite, and pyrite. Covellite, malachite, or azurite are common alteration products.
Related Minerals
Normal chalcocite is monoclinic, but a hexagonal polymorph exists at elevated temperatures. Solid solution with berzelianite (Cu2Se) is common. Similar minerals include stromeyerite (AgCuS) and digenite (Cu2-xS).
▪Argentite (Acanthite) Ag2S
Origin of Name
From the Latin word argentum, which means “silver.”

Hand Specimen Identification
High density, metallic or submetallic luster, dark color, and sectile nature may identify argentite. It can be confused with chalcocite and tetrahedrite which are metallic minerals with a similar color. The arborescent habit seen in Figure 14.279, when present, helps identify this mineral.
Physical Properties
| hardness | 2 to 2.5 |
| specific gravity | 7.1 |
| cleavage/fracture | poor cubic {100}/subconchoidal |
| luster/transparency | metallic/opaque |
| color | lead-gray to black |
| streak | black or shiny black |
Crystallography
Argentite is cubic, a = 4.89, Z = 2; space group $ \small {I \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Wiry, branching, columnar, and massive habit are common. Argentite crystals are cubes, octahedra, dodecahedra, or combinations. Penetration twins are common.
Structure and Composition
In argentite, sulfur atoms are arranged in a distorted body-centered arrangement. Ag atoms occupy 2-fold and 3-fold sites between S.
Occurrence and Associations
Argentite, an important Ag ore mineral, is found in veins associated with other silver minerals, galena, sphalerite, tetrahedrite, and Co-Ni sulfides.
Related Minerals
Argentite has both high-temperature and low-temperature polymorphs. Argentite is the proper name of the high-temperature cubic polymorph, and acanthite is the name of the low-temperature monoclinic polymorph. However, because low-temperature Ag2S is usually twinned, appearing pseudocubic, it is also commonly referred to as argentite. Other related silver minerals include hessite (Ag2Te), petzite (Ag3AuTe), fischesserite (Ag3AuSe2), naumannite (Ag2Se), eucairite (CuAgSe), jalpaite (Ag3CuS2), and aguilarite (Ag4SeS).
▪Pyrite FeS2
Origin of Name
From the Greek word pyr, meaning “fire,” because it sparks when struck by steel. Pyrite has many different appearances and habits. The photos below show some spectacular examples.
Euhedral pyrite crystals are most commonly cubes, such as those seen in the Figures 14.280 and 14.281. Other cubic forms are possible, including octahedra (Figure 14.282). This mineral is often found with other sulfide minerals; Figure 14.283 shows pyrite with galena. Figure 14.284 is a photo of an unusual pyrite form called a sun dollar. Sun dollars develop when pyrite crystallizes in restricted space between layers of high-grade coal.
Hand Specimen Identification
Density, metallic luster, brass-yellow color, and hardness identify pyrite. It is sometimes confused with chalcopyrite and marcasite, both of which have slightly different colors. Pyrite is also called fools gold, but has a more metallic brassy color than gold, and pyrite has a greenish-black streak, while gold has a yellow streak.
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 5.1 |
| cleavage/fracture | poor {100}/subconchoidal |
| luster/transparency | metallic/opaque |
| color | brass-yellow |
| streak | greenish black to green-gray |
Crystallography
Pyrite is cubic, a = 5.42, Z = 4; space group $ \small {I\frac{2_1}{a} \overline{3}\ } $; point group $ \small {\frac{2}{m} \overline{3}}$.
Habit
Pyrite crystals are typically cubes, pyritohedra, or octahedra. Striated faces, combinations of forms, and penetration twinning, sometimes producing iron cross twins, are common.
Structure and Composition
Pyrite‘s structure is closely related to that of NaCl; Fe and S2 alternate in a three-dimensional cubic array. Pyrite commonly contains some Ni and Co as replacements for Fe. Cu, V, Mo, Cr, W, Au, or Tl may also be present.
Occurrence and Associations
Pyrite, the most common and widespread sulfide mineral, is often called fool’s gold. It is an accessory mineral in many igneous, sedimentary, and metamorphic rocks. It is common in all sulfide deposits, associated with a wide variety of ore minerals. It also replaces organic material in coal, wood, or shells.
Related Minerals
Marcasite is an orthorhombic polymorph of pyrite. Pyrite forms solid solutions with vaesite, NiS2, and cattierite, CoS2. Cobaltite, CoAsS, and hauerite, MnS2, are isostructural with pyrite. Many other minerals are isotypical with pyrite. Other related minerals include arsenoferrite, FeAs2; pyrrhotite and mackinawite, both Fe1-xS; greigite, Fe3S4; and smythite, (Fe,Ni)9S11.
▪Cobaltite (Co,Fe)AsS
Origin of Name
From the German word kobold, meaning “goblin,” because early miners found it difficult to mine.


Hand Specimen Identification
Density, luster, whitish color, cleavage, and habit may identify cobaltite. A subtle to quite noticeable pinkish hue (due to tarnishing) help identification. It is sometimes confused with skutterudite.
Figure 14.285 shows cobaltite from a cobalt and silver mining district in northern Ontario. The silver-colored specimen has a slight pinkish tinge. Figure 14.286 shows tarnished cobaltite from Sweden.
Physical Properties
| hardness | 5.5 |
| specific gravity | 6.3 |
| cleavage/fracture | good cubic {100}/uneven |
| luster/transparency | metallic/opaque |
| color | tin-white or silver-white most commonly, occasionally reddish or pink |
| streak | gray-black |
Crystallography
Cobaltite is orthorhombic, a = 5.58, b = 5.58, c = 5.58, Z = 4; space group Pa2c; space group $ \small{Pca2_1} $; point group $ \small{ mm2} $.
Habit
Cobaltite is usually massive. Aggregates may be granular or compact. Rare individual crystals are pseudocubic, similar in form to pyrite.
Structure and Composition
Cobaltite is isostructural with pyrite. Co replaces much of the Fe, and As replaces half the S. Fe and Ni commonly substitute for Co; Sb substitutes for As.
Occurrence and Associations
Cobaltite is commonly found with other cobalt and nickel sulfides, arsenides, and related minerals. Pyrrhotite, chalcopyrite, galena, and magnetite are also associated minerals. It may be veined or disseminated. Cobaltite is also found in a few rare metamorphic rocks.
Related Minerals
Cobaltite forms solid solutions with gersdorffite, NiAsS; ullmanite, NiSbS; and willyamite, (Co,Ni)SbS. Other similar minerals include hollingworthite, (Rh,Pt,Pd)AsS; irarsite, (Ir,Ru,Rh,Pt)AsS; platarsite, (Pt, Rh,Ru)AsS; and tolovkite, IrSbS.
▪Marcasite FeS2
Origin of Name
From Markashitu, an ancient province of Persia.

Hand Specimen Identification
Metallic luster, pale brass-yellow color, and orthorhombic habit identify marcasite. “Cockscomb” groups of twinned crystals are diagnostic. Figure 14.287 shows a mass of golden coxcomb marcasite crystals. Marcasite may be confused with its cubic polymorph, pyrite, if crystals are not well-developed and easily seen.
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 4.9 |
| cleavage/fracture | poor {101}/uneven |
| luster/transparency | metallic/opaque |
| color | white-green to pale bronze yellow, often slightly tarnished |
| streak | grayish black |
Crystallography
Marcasite is orthorhombic, a = 4.44, b = 5.41, c = 3.38, Z = 2; space group $ \small{P \frac{2_1}{n} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Crystal of marcasite are typically tabular, often with curved faces, showing orthorhombic symmetry. They combine to form needle-like groups, sometimes radiating, colloform, globular, reniform, or stalactitic. Twinning often produces cockscomb aggregates.
Structure and Composition
Zigzagging FeS2 chains run parallel to the c-axis; FeS6 octahedra share corners and edges. Marcasite is nearly pure FeS2; traces of Cu may be present.
Occurrence and Associations
Marcasite is a low-temperature mineral found in sulfide veins with lead and zinc minerals or as a replacement mineral in limestones or shale. Common associates are galena, pyrite, chalcopyrite, calcite, and dolomite.
Related Minerals
Pyrite is a more stable polymorph of marcasite. Isostructural minerals include hastite, CoSe2; ferroselite, FeSe2; frohbergite, FeTe2; kullerudite, NiSe2; and mattagamite, CoTe2.
▪Arsenopyrite FeAsS
Origin of Name
Named for its composition.

Hand Specimen Identification
Metallic luster, silver-white to gray color, and crystal shape help identify arsenopyrite. It may be confused with marcasite, pyrite, or skutterudite, but color distinguishes it from the first two, and crystal shape and habit from the latter. Arsenopyrite sometimes smells like garlic when struck with a hammer or other hard object. Figure 14.288 shows arsenopyrite with calcite.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 6.1 |
| cleavage/fracture | good (101)/uneven |
| luster/transparency | metallic/opaque |
| color | silver-white to gray |
| streak | black |
Crystallography
Arsenopyrite is monoclinic, a = 5.76, b = 5.69, c = 5.785, β = 112.2°, Z = 4; space group $ \small{P \frac{2_1}{c}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Prismatic, striated crystals of arsenopyrite are typical. Penetration, contact, and cyclic twins are common. It may be disseminated, massive, or granular.
Structure and Composition
Arsenopyrite‘s structure is similar to the atomic arrangement in marcasite with half the S replaced by As. FeAs3S3 octahedra share vertices and edges. As:S ratios vary slightly, but arsenopyrite is always close to FeAsS in composition. Minor Co and Bi may replace Fe and other elements may be present in trace amounts.
Occurrence and Associations
Arsenopyrite, the most abundant and widespread arsenic mineral, is found in Fe, Cu, Sn, Co, Ni, Ag, Au, and Pb ores. It occurs in veins, pegmatites, contact aureoles, or as disseminations in low- to medium-grade metamorphic rocks. Common associated minerals include chalcopyrite, pyrite, sphalerite, cassiterite, and gold and silver minerals.
Related Minerals
Arsenopyrite forms solid solutions with glaucodot, (Co,Fe)AsS. It is isotypical with marcasite, FeS2, and with gudmundite, FeSbS. Other related arsenic minerals include lautite, CuAsS; osarsite, (Os,Ru)AsS; and ruarsite, RuAsS.
▪Skutterudite (Co,Ni)As3
Origin of Name
From the type locality at Skutterude, Norway.

Hand Specimen Identification
The skutterudite series contains a number of related minerals; they have similar properties and are difficult to tell apart. Density, luster, and color help with identification, but chemical or X-ray analysis may be needed. Skutterudite is sometimes confused with arsenopyrite or cobaltite. Figure 14.289 shows an example of skutterudite ore from Morocco.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 6.1 to 6.8 |
| cleavage/fracture | good (101)/uneven |
| luster/transparency | metallic/opaque |
| color | tin-white to silver-gray |
| streak | black |
Crystallography
Skutterudite is cubic, a = 8.20, Z = 8; space group $ \small {F \overline{4}3m} $; point group $ \small {\overline{4}3m} $.
Habit
Cubes and octahedra are common forms. Skutterudite usually forms dense to granular aggregates.
Structure and Composition
In skutterudite, Co and Ni, in octahedral coordination, are linked to square AsS4 groups. Fe and Bi may substitute for Co or Ni. Some As may be missing or replaced by S or Sb.
Occurrence and Associations
Skutterudite is a vein mineral associated with other Co and Ni minerals such as cobaltite or niccolite. Other associated minerals include arsenopyrite, native silver, silver sulfosalts, native bismuth, calcite, siderite, barite, and quartz.
Related Minerals
The three important members of the skutterudite series are skutterudite, (Co,Ni)As3; smaltite, (Co,Ni)As3; and chloanthite, (Ni,Co)As3-x. Linnaeite, Co3S4, is closely related.
▪Stibnite Sb2S3
Origin of Name
From the Greek word stibi, a name originally used by Pliny, a first-century Greek encyclopedist and scientist.


Hand Specimen Identification
Stibnite is characterized by its softness, perfect cleavage in one direction, black streak, bladed or columnar habit, and lead-gray color. Euhedral crystals often form in parallel or subparallel masses such as seen in Figure 14.290. Figure 3.22 is a photo of a similar cluster, but it is huge. It is nearly a meter across and weighs 90 kg. This habit and gray color are diagnostic for stibnite. When massive (Figure 14.291), stibnite may be confused with any of a number of other gray minerals.
Physical Properties
| hardness | 2 |
| specific gravity | 4.6 |
| cleavage/fracture | one perfect (010)/subconchoidal |
| luster/transparency | metallic/opaque |
| color | lead-gray |
| streak | lead-gray to black |
Crystallography
Stibnite is orthorhombic, a = 11.12, b = 11.30, c = 3.84, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Prismatic striated metallic-gray crystals, often slender, long, and curved, typify stibnite. Faces show striations; terminating faces may be steep. Typically, stibnite is found in aggregates containing granular to coarse columns or needles.
Structure and Composition
Stibnite‘s structure contains zigzagging chains of Sb2S3 parallel to the c-axis Small amounts of other metals may replace Sb. Fe, Pb, and Cu are the most common impurities. Ag, Au, Zn, and Co may also be present in trace amounts.
Occurrence and Associations
Stibnite is found in hydrothermal veins, in replacement deposits, and more rarely, in hot spring deposits. Typical associates include orpiment, realgar, cinnabar, galena, sphalerite, pyrite, barite, and sometimes gold.
Related Minerals
Bismuthinite, Bi2S3, and guanajuatite, Bi2Se3, are isostructural with stibnite.
▪Tetrahedrite Cu12Sb4S13
Origin of Name
Named for its typical crystal shape.


Hand Specimen Identification
Crystals shaped like tetrahedra (Figure 14.292), lack of cleavage, and silver to black color help identify a number of minerals that belong to the tetrahedrite series, Cu12(Sb,As)4S13. The different minerals, however, cannot be told apart without chemical analysis or X-ray study. Besides tetrahedra, other cubic forms are possible; Figure 14.293, for example, is a photo of cube-shaped tetrahedrite crystals.
Physical Properties
| hardness | 3 to 4.5 |
| specific gravity | 4.5 to 5.1 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | metallic/opaque |
| color | silver or grayish black to black |
| streak | brown to black |
Crystallography
Tetrahedrite is cubic, a = 10.34, Z = 2; space group $ \small {I\overline{4}3m} $; point group $ \small {\overline{4}3m} $.
Habit
Tetrahedrite crystals are typically tetrahedra, sometimes with modifying faces. Penetration twins are common. Crystal aggregates may be massive or granular.
Structure and Composition
The structure of tetrahedrite is similar to that of sodalite. CuS4 tetrahedra share corners. Sb or As occupy openings between the tetrahedra. Fe, Zn, Pb, Ag, and Hg may replace Cu. Complete solid solution exists between end members tetrahedrite, Cu12Sb4S13, and tennantite, Cu12As4S13.
Occurrence and Associations
Tetrahedrite, one of the most common sulfosalts, is found in veins and in replacement deposits. Associated minerals include chalcopyrite, sphalerite, galena, pyrite, argentite, and many other minerals.
Varieties
Freibergite is a Ag-rich variety of tetrahedrite. Schwatzite is an Hg-rich variety.
Related Minerals
Tennantite, Cu12As4S13, is isostructural with tetrahedrite. Other related minerals include germanite, Cu3(Ge,Fe)S4; colusite, Cu3(As,Sn,Fe)S4; and sulvanite, Cu3VS4.
▪Pyrargyrite (Ruby Silver) Ag3SbS3
Origin of Name
From the Greek words meaning “fire” and “silver,” relating to its color and composition.


Hand Specimen Identification
Distinctive red color, translucence, and density may identify pyrargyrite. It is commonly found with other silver-bearing minerals.
Pyrargyrite may be confused with proustite but generally has a ruby-red color, while proustite is more vermillion. It is occasionally mistaken for cuprite which is typically brownish red to purple. Figures 14.294 and 14.295 show two examples.
Physical Properties
| hardness | 2 |
| specific gravity | 5.85 |
| cleavage/fracture | good {101}/subconchoidal |
| luster/transparency | adamantine/translucent |
| color | ruby-red to deep red-purple to purple |
| streak | red to purple |
Properties in Thin Section
Pyrargyrite is uniaxial (-), ω = 3.08, ε = 2.88, δ = 0.20.
Crystallography
Pyrargyrite is hexagonal (trigonal), a = 11.06, c = 8.73, Z = 6; space group R3c; point group 3m.
Habit
Pyrargyrite most commonly forms trigonal striated prismatic crystals. Simple, repeated, and cyclic twins are common. Aggregates may be massive or granular.
Structure and Composition
Pyrargyrite‘s structure is composed of three S bonded to Sb to form pyramids. Ag occupies large sites between the pyramids. Cu may replace Ag; As and Bi may replace Sb.
Occurrence and Associations
Pyrargyrite is found in low-temperature veins and in replacement deposits. Associated minerals include native silver, argentite, tetrahedrite, galena, sphalerite, carbonates, and quartz.
Related Minerals
Pyrargyrite has two polymorphs: pyrostilpnite and xanthoconite. Proustite, Ag3AsS3, is isostructural with pyrargyrite, but solid solutions are limited. Both pyrargyrite and proustite are called ruby silvers.
▪Orpiment As2S3
Origin of Name
From the Latin word aurum and pigmentum, meaning “golden paint,” referring to its color.


Hand Specimen Identification
Orpiment is one of the few yellow non-metallic minerals. Its foliated structure and hardness help identify it. When visible, perfect cleavage distinguishes it from sulfur – which sometimes has a similar appearance and habit. Odor, too (sulfur smells) helps make the distinction.
Orpiment has a yellow color, but it is almost always found with realgar, a red-pink mineral with just about the same composition. Figure 14.296 shows orpiment (yellow) with minor realgar (pinkish). The specimen seen in Figure 14.297 is mostly realgar with a lesser amount of orpiment on the sides.
Physical Properties
| hardness | 1.5 to 2 |
| specific gravity | 3.49 |
| cleavage/fracture | one perfect (010)/even or sectile |
| luster/transparency | resinous, also pearly on cleavage face/translucent |
| color | lemon-yellow to orange-yellow |
| streak | pale yellow to yellow |
Properties in Thin Section
Orpiment is biaxial (-) a = 2.40 , β = 2.81, δ = 3.02, 2V = 76°.
Crystallography
Orpiment is monoclinic, a = 11.49, b = 9.59, c = 4.25, β = 90.45°, Z = 4; space group $ \small{R3c}$; point group $ \small{3m}$.
Habit
Rare crystals of orpiment are small, tabular, or prismatic, often poorly formed. Columnar or foliated aggregates are common.
Structure and Composition
In orpiment, AsS3 pyramids share edges, producing 6-member rings. Crumpled layers of rings are stacked on top of each other.
Occurrence and Associations
Orpiment, a rare mineral found in hot spring deposits and some gold deposits, is commonly associated with realgar. Other associated minerals include stibnite, native arsenic, calcite, barite, and gypsum.
Related Minerals
Getchellite, AsSbS3, is similar in structure to orpiment. Realgar, AsS, is closely related in composition.
▪Realgar AsS
Origin of Name
From the Arabic phrase rahj al-ghar, meaning “powder of the mine.”


Hand Specimen Identification
Association with orpiment, resinous luster, orange-red streak, and red color identify realgar. It is sometimes confused with cinnabar, cuprite, or hematite because of its red color.
Figure 14.298 shows vermillion-colored realgar on top of galena. Minor orangish orpiment is also present. Figure 14.299 shows redder realgar with yellow orpiment and gray galena. See also Figure 14.297, above.
Physical Properties
| hardness | 1.5 to 2 |
| specific gravity | 3.56 |
| cleavage/fracture | good (010)/conchoidal |
| luster/transparency | resinous/transparent to translucent |
| color | red to orange |
| streak | red to orange |
Properties in Thin Section
Realgar is biaxial (-), α = 2.538 , β = 2.864, γ = 2.704, δ = 0.166, 2V = 41°.
Crystallography
Realgar is monoclinic, a = 9.29, b = 13.53, c = 6.57, β = 106.55°, Z = 4; space group $ \small{P \frac{2_1}{n} } $; point group $ \small{\frac{2}{m} } $.
Habit
Realgar may form short prismatic crystals having vertical striations. It is often massive granular or forms as earthy crusts.
Structure and Composition
Realgar contains uneven rings of As4S4 that form layers in the structure. The As atoms, lying alternately above and below the plane of the S atoms, are covalently bonded to As in adjacent layers.
Occurrence and Associations
Realgar is associated with lead, gold, and silver ores. Associated minerals include orpiment, other arsenic minerals, and stibnite.
Related Minerals
Orpiment, As2S3, is closely related in composition.
4 Halide Minerals
| Halides halite NaCl sylvite KCl chlorargyrite AgCl atacamite Cu2Cl(OH)3 fluorite CaF2 cryolite Na3AlF6 |
Fluorine and other halogens generally form nearly pure ionic bonds of moderate strength. Consequently, they bond with alkali and alkali earth elements to make chlorides, fluorides, bromides, and other salts referred to collectively as halides. Due to the generally large cation size and the nature of the bonding, halide structures tend to be simple with high symmetry. At high temperatures, some halides exhibit mutual solubility, but under normal Earth-surface conditions most are usually close to end-member composition. Halite and fluorite are the most common halide minerals, but others may be locally abundant.
For more general information about halides, see Section 7.4.5 in Chapter 7.
▪Halite NaCl
Origin of Name
From the Greek word halos, meaning “salt.”
Hand Specimen Identification
Its softness (H = 2.5), transparency or white color, salty taste, and cubic cleavage identify halite. Classic specimens are clear transparent white cubes, like those seen below in Figure 14.300. Commonly, however, the cubes are translucent (instead of transparent) and develop in clusters (Figure 14.301). Halite may be deliquescent (absorb water and turn into a liquid under extreme humidity.
Halite comes in just about any color; Figures 14.302 and 14.303 show pink and green varieties. The pink halite crystals in Figure 14.322 are hopper crystals, which means that their edges grew faster than the centers of faces.
Halite is similar to sylvite, but is quite easily distinguished by its less bitter taste. It can also be confused with other soft clear or white minerals such as cryolite.
Physical Properties
| hardness | 2.5 |
| specific gravity | 2.16 |
| cleavage/fracture | perfect cubic {100}/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless and transparent but, if impure, may be shades of red, blue, purple, or other colors |
| streak | white |
Properties in Thin Section
Halite has low relief, perfect cubic cleavage, and is colorless in thin section. Isotropic, n = 1.5446.
Crystallography
Halite is a cubic mineral, a = 5.6404, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Cubic crystals, sometimes granular, sometimes massive, and sometimes in clusters, characterize halite. Cubic cleavage is pronounced.
Structure and Composition
Closest packed Na+ and Cl– alternate in a face-centered cubic arrangement. Both ions are in perfect octahedral coordination. Sylvite (KCl), galena (PbS), periclase (MgO) and several other minerals are isostructural with halite.

Occurrence and Associations
Halite, a rock-forming mineral, occurs in salt flats, in sedimentary beds, in salt domes, and as deposits from volcanic gasses. Figure 14.304 shows halite deposited along the shores of the Dead Sea. Halite is, by far, the most common evaporite mineral. Associated minerals include many other salts, gypsum, calcite, sylvite, anhydrite, sulfur, and clay.
Related Minerals
Galena, PbS; alabandite, MnS; periclase, MgO; sylvite, KCl; carobbiite, KF; and chlorargyrite, AgCl are all isostructural with halite.
▪Sylvite KCl
Origin of Name
Named after Francois Sylvius de le Boe, a 17th century Dutch chemist.


Hand Specimen Identification
Sylvite shares most properties with halite. It is soft (H = 2), generally white or clear, and displays well-developed cubic cleavage. It is distinguished from halite by a more bitter taste. Sylvite may be deliquescent (absorb water and turn into a liquid under extreme humidity.
Figure 14.305 is a photo of a sylvite sample that can only be distinguished from halite by its taste. Figure 14.306 shows orange sylvite with clear halite, a common combination. This association and orange color identify the mineral.
Physical Properties
| hardness | 2 |
| specific gravity | 1.99 |
| cleavage/fracture | perfect cubic {100}/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless, but may be white, or shades of yellow, blue, or red caused by impurities |
| streak | white |
Properties in Thin Section
Sylvite has low relief, perfect cubic cleavage, and is colorless in thin section. Isotropic, n = 1.490.
Crystallography
Sylvite is cubic, a = 6.29, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Sylvite crystals are typically cubes, often with modifying octahedra. Massive or granular aggregates are typical.
Structure and Composition
Halite (NaCl), sylvite (KCl), galena (PbS), periclase (MgO) and several other minerals are isostructural. In sylvite, K+ and Cl– are arranged in a face centered cubic manner. Only very minor solid solution exists between the two salts.
Occurrence and Associations
Sylvite is rarer than halite but has the same origin, associates, and occurrences (see halite occurrences).
Related Minerals
Sylvite and halite are isostructural with a number of other minerals including galena (PbS) and periclase (MgO). Associated potassium salts include carnallite, KMgCl3•6H2O; kainite, KMg(Cl,SO4)•nH2O; and polyhalite, K2Ca2Mg(SO4)4•2H2O.
▪Chlorargyrite AgCl
Origin of Name
From the Greek words chlor and argyros, meaning “green” and “silver.”

Hand Specimen Identification
A waxy or resinous appearance and typical light green, gray, or brown color characterize chlorargyrite. It is soft (H = 2.5) and has relatively high specific gravity (5.55). Tarnished appearance and occurrence with other silver minerals aid identification. It is generally very fine-grained, however, sometimes making identification difficult.
Physical Properties
| hardness | 2.5 |
| specific gravity | 5.55 |
| cleavage/fracture | poor {100}/subconchoidal |
| luster/transparency | adamantine, resinous or waxy/translucent, rarely transparent |
| color | colorless if unoxidized, otherwise pale green, pearl-gray, or brown |
| streak | white |
Properties in Thin Section
Chlorargyrite is isotropic, n = 2.071.
Crystallography
Chlorargyrite is cubic, a = 5.55, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$./m.
Habit
Rare chlorargyrite crystals are cubic. Chlorargyrite is usually massive or columnar.
Structure and Composition
Chlorargyrite is isostructural with halite (NaCl), sylvite (KCl), galena (PbS), periclase (MgO) and several other minerals. Br, F, and I may substitute for Cl in chlorargyrite. Hg or Fe may be present in small amounts.
Occurrence and Associations
Chlorargyrite is a secondary silver mineral found in the oxidized zones of silver deposits. Associated minerals are many, including native silver.
Varieties
Embolite is a Br-rich variety of chlorargyrite.
Related Minerals
Chlorargyrite forms complete solid solutions with bromargyrite, AgBr. It is isostructural with a number of other minerals, including halite (NaCl), sylvite (KCl), galena (PbS), periclase (MgO) and several other minerals. Other related minerals include iodargyrite, AgI.
▪Atacamite Cu2Cl(OH)3
Origin of Name
From Atacama, a province in Chile.


Hand Specimen Identification
Atacamite occurs in various hues. It typically has a bright emerald-green, olive-green, or blackish-green color. It may form as fine-grained granular crystal aggregates or in clusters of prismatic crystals, sometimes radiating. Occurrence with other secondary copper minerals aids identification.
Physical Properties
| hardness | 3 to 3.5 |
| specific gravity | 3.76 |
| cleavage/fracture | perfect {010}, fair {101} |
| luster/transparency | adamantine/transparent to translucent |
| color | various shades of green |
| streak | green |
Properties in Thin Section
Atacamite is biaxial (-), α = 1.831 , β = 1.861, γ = 1.880, δ = 0.049, 2V = 75°.
Crystallography
Atacamite is orthorhombic, a = 6.02, b = 9.15, c = 6.85, Z = 4; space group $ \small{P \frac{2_1}{n} \frac{2_1}{m} \frac{2_1}{a}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Slender, striated prisms or needles are typical for atacamite. Massive, granular, or fibrous aggregates are common.
Structure and Composition
Atacamite contains two kinds of octahedra: CuCl(OH)5 and CuCl2(OH)4. Mn commonly substitutes for Cu.
Occurrence and Associations
Atacamite occurs in the oxidized zones of copper deposits and in sands. It is associated with malachite, cuprite, and a number of rare secondary copper minerals.
▪Fluorite CaF2
Origin of Name
From the Latin word fluere, meaning “to flow,” referring to the ease with which it melts.



Hand Specimen Identification
Fluorite comes in just about any color, and may be clear. If it is purple, identification is generally straightforward. It is relatively soft (H = 4) and forms distinctive cubic (Figure 14.310) or, less commonly, octahedral (Figure 14.311) crystals. It always displays excellent cleavage. When uncolored, fluorite is sometimes confused with calcite or quartz, but may be distinguished by hardness and habit.
Purple fluorite is most common; Figures 14.310 and 14.311 show examples. Figure 4.3 (Chapter 4) is another example; it contains purple fluorite on top of white calcite and brown scheelite. Figure 14.312 shows rarer blue crystals, and Figure 9.91 is a photo of green fluorite with tan calcite and gray galena.
Physical Properties
| hardness | 4 |
| specific gravity | 3.18 |
| cleavage/fracture | perfect octahedral {111}/ conchoidal and splintery |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless to light green, blue-green, yellow, or purple; more rarely can also be white, brown, or rose |
| streak | white |
Properties in Thin Section
Fluorite is usually colorless in thin section; occasionally, it appears light purple or green. Octahedral cleavage and low refractive index distinguish it from other clear isotropic minerals. Isotropic, n = 1.434.
Crystallography
Fluorite is cubic, a = 5.463, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Cubic or octahedral crystals are common; octahedral cleavage fragments may appear to be crystals. Penetration twins are common. Fluorite may be massive or granular.
Structure and Composition
The cubic unit cell has Ca2+ ions in 8-fold coordination at the corners and in the middle of faces.
F– ions are located in tetrahedral coordination within the cell just inside each of the four corners. Fluorite is generally close to end-member composition. Minor Y, Ce, and other rare earths may substitute for Ca. Cl, Sr, Ba, Fe, and Na may be present in small amounts.
Occurrence and Associations
Fluorite is common and widespread. It typically occurs in veins where it is associated with quartz, calcite, galena, barite, and a number of other minerals. In some carbonate-hosted ore deposits, fluorite is a replacement or fracture filling mineral associated with pyrrhotite, galena, and pyrite. It is also found as an accessory mineral in limestones and in igneous and metamorphic rocks.
Related Minerals
Fluorite is isostructural with thorianite, ThO2; cerianite, (Ce,Th)O2; and uraninite, UO2. It is closely related to sellaite, MgF2, and frankdicksonite, BaF2.
▪Cryolite Na3AlF6
Origin of Name
From the Greek words for “ice” and “stone,” referring to its icy appearance.

Hand Specimen Identification
A pearly/greasy luster, white color, common coarsely granular habit, and softness (H = 2.5) help identify cryolite. It is, however, a rare mineral that is quite similar to other soft white and translucent minerals. Most cryolite specimens come from a single location in Greenland.
Figure 14.313 is a photo of cryolite from Mont Saint-Hilaire, 40 km east of Montréal. Mont Saint-Hilaire and the nearby Poudrette Quarry are known sources of many unique mineral species.
Physical Properties
| hardness | 2.5 |
| specific gravity | 2.97 |
| cleavage/fracture | none/uneven; cubic parting |
| luster/transparency | pearly, greasy, vitreous/transparent to translucent |
| color | colorless to snow-white |
| streak | white |
Properties in Thin Section
Cryolite is biaxial (+), α = 1.3385 , β = 1.3389, γ = 1.3396, δ = 0.0011, 2V = 43°.
Crystallography
Cryolite is monoclinic, a = 5.46, b = 5.60, c = 7.80, β = 90.18°, Z = 2; space group $ \small{P \frac{2_1}{n} } $; point group $ \small{\frac{2}{m} } $.
Habit
Large visible cryolite crystals are rare but may be pseudocubic. Aggregates are massive, lamellar, or columnar, often exhibiting pseudocubic parting.
Structure and Composition
In cryolite, both Na and Al are coordinated to six F. Na octahedra are distorted. F occupies a tetrahedral site, coordinated to three Na and one Al.
Occurrence and Associations
Cryolite is a rare mineral. The most significant samples are from Greenland, where it is in ore deposits hosted by granitic rocks. Associated minerals include quartz, K-feldspar, siderite, galena, sphalerite, and chalcopyrite, and less commonly other sulfides, wolframite, cassiterite, fluorite, and columbite.
Related Minerals
Cryolite has a high-temperature cubic polymorph.
5 Oxide Minerals
5.1 Tetrahedral and Octahedral Oxides
|
Tetrahedral Oxides Octahedral Oxides |
Similar to the sulfides, mineralogists divide oxide minerals into those having metal ions only in tetrahedral or only in octahedral coordination, and those in which the ions occupy sites with mixed or unusual coordinations. Zincite, a rare mineral, is the only known example of a purely tetrahedral oxide, but more than a dozen octahedral oxides are known. At high temperatures, Mg-, Fe-, and Ti-oxides form stable solid solutions. At low temperatures, most intermediate compositions are unstable; consequently, exsolution is common.
For more general information about oxides, see Section 9.2.3 in Chapter 9.
▪Zincite ZnO
Origin of Name
Zincite is named for its composition.

Hand Specimen Identification
Zincite is identified by its association with other Zn-minerals (most commonly willemite and franklinite), its orange-yellow streak, and its common red color. Other colors are possible, making identification sometimes difficult. The specimen in Figure 14.314 comes from a famous Zn-mineral collecting site in New Jersey.
Physical Properties
| hardness | 4 to 4.5 |
| specific gravity | 5.4 to 5.7 |
| cleavage/fracture | perfect basal (001)/subconchoidal |
| luster/transparency | subadamantine/translucent |
| color | orange, yellow to deep red; rarely other colors |
| streak | orange-yellow |
Properties in Thin Section
Zincite is uniaxial (+), ω = 2.013, ε = 2.029, δ = 0.016.
Crystallography
Zincite is a hexagonal mineral, a = 3.25, c = 5.19, Z = 2; space group $ \small{{P6_3}mc}$ ; point group $ \small{6mm}$.
Habit
Zincite specimens are typically massive, platy, or granular. Rare crystals are hexagonal prisms terminated by pyramids and pedions.
Structure and Composition
Zincite has the same structure as wurtzite; Zn is hexagonal closest packed. Mn and minor Fe may substitute for Zn.
Occurrence and Associations
Zincite is a rare mineral, primarily found at Franklin, New Jersey. Associated minerals include calcite, dolomite, franklinite, and willemite.
Related Minerals
Zincite is isostructural with wurtzite, ZnS; enargite, Cu3AsS4; and greenockite, CdS.
▪Rutile TiO2
Origin of Name
From the Latin word rutilas, meaning “red.”



Hand Specimen Identification
Red-brown to reddish-black color and adamantine luster are keys to rutile identification. When present, tetragonal prismatic crystals aid identification. Figures 14.315 and 14.316 show typical anhedral examples.

Reddish rutile, such as the rutile seen in Figure 14.315, is significantly more common than black rutile (Figure 14.316) Most rutile occurrences are very fine-grained, but when coarse and euhedral, rutile may be in spectacular specimens, typically crystal clusters (Figure 14.317). Rutile also, sometimes, develops diagnostic cyclic twins like those seen in Figure 14.318.
Physical Properties
| hardness | 6 to 6.5 |
| specific gravity | 4.24 |
| cleavage/fracture | good prismatic {100} and {110}/subconchoidal |
| luster/transparency | adamantine, submetallic/transparent to translucent |
| color | red, red-brown to black |
| streak | pale or light brown |
Properties in Thin Section
Rutile appears deep red, red-brown, or yellow-brown in thin section. The strong color may mask its extreme birefringence. Relief is very high. Uniaxial (+), ω = 2.61, ε = 2.90, δ = 0.29.
Crystallography
Rutile is a tetragonal mineral, a = 4.59, c = 2.96, Z = 2; space group $ \small{P \frac{4_2}{m} \frac{2_1}{n} \frac{2}{m}} $; point group $ \small{ \frac{4}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Rutile may be massive but more commonly forms stubby to acicular tetragonal crystals. Striated prismatic crystals, terminated by prisms and often twinned, are common.
Structure and Composition
In rutile, distorted TiO6 octahedra share edges to form chains. Chains are connected by corner-sharing octahedra. Each O is in triangular coordination, bonded to three Ti. Fe, Ta, Nb, V, Sn, and Cr may be present as substitutions. Minerals with similar structures to rutile include cassiterite, SnO2; pyrolusite, MnO2; plattnerite, PbO2; and stishovite, SiO2.
Occurrence and Associations
Rutile, although not particularly abundant, is widespread. It is found typically as small grains in intermediate to mafic igneous rocks, in some metamorphic rocks, in veins, in pegmatites, and in some sediments. It sometimes occurs as needles in quartz. Associated minerals include quartz, feldspar, ilmenite, and hematite.
Varieties
Sagenite is the name given to rutile that exists as needled patches within other minerals such as quartz and pyroxene.
Related Minerals
Rutile has several polymorphs; most important are anatase and brookite.
▪Periclase MgO
Origin of Name
From the Greek words peri and klasis, meaning “even” and “fracture,” referring to its perfect cubic cleavage.


Hand Specimen Identification
Clear or light color, equant granular crystals, cubic cleavage (if visible), and occurrence in marbles help identify periclase. Most periclase occurs as very fine grains in metadolomites.
These two photos show examples of periclase. In these photos, white dolomite surrounds green and brown periclase crystals. Periclase (Mg-oxide) commonly alters to brucite (Mg-hydroxide); the periclase crystals in Figure 14.320 have brucite rims.
Physical Properties
| hardness | 5.5 |
| specific gravity | 3.56 |
| cleavage/fracture | perfect {100}, poor {111}/ uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless or gray, rarely yellowish or brown |
| streak | white |
Properties in Thin Section
Periclase is colorless in thin section, has high relief and cubic cleavage. Isotropic, n = 1.736.
Crystallography
Periclase is cubic, a = 4.21, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Typically periclase crystals are cubes or octahedra. Coarse or granular masses are common.
Structure and Composition
In periclase, Mg and O alternate in a three-dimensional cubic framework. Fe, Zn, and minor Mn may substitute for Mg. Halite (NaCl), sylvite (KCl), galena (PbS), periclase (MgO) and several other minerals are isostructural.
Occurrence and Associations
Periclase is a high-temperature mineral found in metamorphosed carbonates. It is typically in contact aureoles, associated with calcite, forsterite, diopside, and a number of other Ca- and Ca-Mg-silicates.
Related Minerals
Periclase is isostructural with halite (NaCl), sylvite (KCl), galena (PbS), periclase (MgO) and several other minerals.
▪Hematite Fe2O3
Origin of Name
From the Greek word haimatos, meaning “blood,” a reference to its color when powdered.



Hand Specimen Identification
Hematite has variable appearance, but high density, dark color, and a characteristic red streak are keys to identification. If red, hematite may sometimes be confused with cinnabar, another red mineral.
The photos seen above are typical. Figure 14.321 is a photo of hematite displaying variable reddish-brown hues. Figure 14.322 is a photo of a crystal displaying the mineral’s hexagonal symmetry. Figure 14.323 is a photo of botryoidal hematite. Hematite‘s luster is variable from earthy to metallic. Figure 14.324, below, shows a particularly metallic variety of the mineral called specular hematite.
Physical Properties
| hardness | 5.5 -6.5 |
| specific gravity | 4.9 to 5.3 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | submetallic/translucent to opaque |
| color | steel-gray, red-brown to black |
| streak | red |
Properties in Thin Section
Hematite is usually opaque; when thin, it may have a deep red color. Uniaxial (-), ω = 3.22, ε = 2.96, δ = 0.28.
Crystallography
Hematite crystals are trigonal. a = 5.04, c = 13.76, Z = 6; space group $ \small {R\overline{3}\frac{2}{c}}$; point group $ \small {\overline{3}\frac{2}{m}}$.
Habit
Hematite exhibits many habits. Aggregates may be massive, in rosettes, botryoidal, reniform, micaceous and foliated, or earthy. Individual crystals are tabular, with many forms making up the faces. Twins are common.
Structure and Composition
Hematite‘s structure is similar to that of corundum. Closest packed O2- forms hexagonal layers; Fe3+ occupies two of three interlayer octahedral sites. FeO6 octahedra, linked by edge sharing, form 6-sided rings. Ti, Fe, Al, and Mn may replace Fe in small amounts.
Occurrence and Associations
Hematite is a common mineral in many kinds of rocks. It is found in red sandstones, iron formations, and their metamorphic equivalents; as an accessory mineral in igneous rocks; as coatings and nodules; and in hydrothermal veins. Hematite is also associated with altered zones of ore deposits where it is typically secondary, forming after magnetite, Fe-sulfides, or Fe-silicates.


Varieties
Specular hematite, also called specularite, is the name given to micaceous or massive hematite exhibiting a splendent metallic luster (Figure 14.324). Martite is the name given to hematite pseudomorphs after magnetite (Figure 14.325). Ocher is a red, earthy form of hematite.
Related Minerals
Hematite has a rare polymorph, maghemite. Isostructural minerals include corundum, Al2O3; eskolaite, Cr2O3; and karelianite, V2O3. Turgite, 2Fe2O3•nH2O, and limonite, Fe2O3•nH2O, are equivalent to hydrated hematite with variable water content. Other related minerals include goethite and lepidocrocite, both FeO(OH), and bixbyite, Mn2O3.
▪Corundum Al2O3
Origin of Name
Derived from kauruntaka, the Indian name for the mineral.


Hand Specimen Identification
Corundum is identified primarily by its hardness and crystal shape. Figure 14.326 shows typical hexagonal cross sections. Figure 14.327 is a photo of diagnostic subhedral to euhedral barrel-shaped crystals. Luster, specific gravity, and habit aid identification.
Corundum comes in many colors, but the red and brown colors seen in the figures above are the most common. Other colors are, however, well known. Figure 3.41 shows some examples. Many colored varieties have gem names (see Figures 14.328, 14.329, and 14.330, below).
Physical Properties
| hardness | 9 |
| specific gravity | 3.9 to 4.1 |
| cleavage/fracture | none/uneven; rectangular parting |
| luster/transparency | adamantine/transparent to translucent |
| color | shades of brown, blue, red, pink, colorless, variable |
| streak | white |
Properties in Thin Section
In thin section, corundum is typically colorless. Some varieties are pale pink, green, blue, or yellow. It has extremely high relief and is weakly birefringent. Uniaxial (-), ω = 1.768, ε = 1.760, δ = 0.008.
Crystallography
Corundum belongs to the trigonal crystal system. a = 4.95, c = 13.78, Z = 6; space group $ \small {R\overline{3}\frac{2}{c}}$; point group $ \small {\overline{3}\frac{2}{m}}$.
Habit
Hexagonal corundum crystals may be tabular or prismatic. Multiple pyramidal forms combined with pinacoids give a tapering or barrel-shaped appearance. Euhedral individual crystals are rare; corundum is usually massive or granular.
Structure and Composition
The corundum structure is identical to that of hematite (see hematite structure, above). Minor amounts of Fe, Ti, Cr, Ni, and Mn replace Al.
Occurrence and Associations
Corundum is an accessory mineral in metamorphosed carbonates and sediments, in some Al-rich igneous rocks, and in placers. Massive corundum with iron oxides, forming emery deposits, is found in carbonate skarns.



Varieties
Ruby (always red) and sapphire (typically blue but sometimes other colors) are gem varieties of corundum. Figures 14.328 and 14.329 show crystals of ruby and sapphire. Figure 14.330 is a photo of the Star of India, one of the largest star sapphire cabochons in the world.
Related Minerals
Corundum is isostructural with hematite (Fe2O3), ilmenite (FeTiO3) eskolaite (Cr2O3) and karelianite (V2O3).
▪Ilmenite FeTiO3
Origin of Name
Named after the Ilmen Mountains in Russia.


Hand Specimen Identification
Density and black color help identify ilmenite. It is distinguished from magnetite by its lack of strong magnetism and from hematite by its (not red) streak. Ilmenite is occasionally confused with chromite.
The photos here show typical ilmenite crystals. Ilmenite is generally in the form of small crystals, like those in Figure 14.331. The larger crystal seen in Figure 14.332 is quite exceptional. Figure 9.55 shows another exceptional example, a large hexagonal ilmenite crystal from Quebec.
Physical Properties
| hardness | 5.5 to 6 |
| specific gravity | 4.5 to 5 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | metallic/opaque |
| color | iron-black |
| streak | brownish red to black |
Crystallography
Ilmenite crystals are trigonal. a = 5.08, c = 14.08, Z = 6; space group $ \small{R3}$; point group $ \small{3}$.
Habit
Ilmenite crystals are tabular or prismatic, often showing rhombohedral forms, and commonly twinned. The most common occurrences are massive, granular, compact, scaly, or appearing as skeletal crystals.
Structure and Composition
Ilmenite is isostructural with hematite (Fe2O3), corundum (Al2O3) eskolaite (Cr2O3) and karelianite (V2O3). Ilmenite may contain excess Fe replacing Ti, forming limited solid solutions with hematite. Mg and Mn may replace Fe.
Occurrence and Associations
Ilmenite, a common vein mineral, is found as masses in igneous rocks, in pegmatites, as an accessory in high-grade metamorphic rocks, and is present in black sands where associated minerals include quartz, hematite, magnetite, rutile, zircon, monazite, and other dense minerals.
Related Minerals
Geikielite, MgTiO3, and pyrophanite, MnTiO3, form complete solid solutions with ilmenite.
▪Cassiterite SnO2
Origin of Name
From the Greek word kassiteros, meaning “tin.”
Hand Specimen Identification

Cassiterite is recognized by crystal shape, dark color but white streak, high specific gravity, indistinct prismatic cleavage, and adamantine luster. Thin crystals may be translucent. Cassiterite has historically been the most important tin ore mineral. Because of its adamantine luster, it is sometimes valued as a gemstone. It may be confused with rutile if it has a deep reddish-brown-black color.
Physical Properties
| hardness | 6 to 7 |
| specific gravity | 7.0 |
| cleavage/fracture | good {100}, poor {111}/ subconchoidal |
| luster/transparency | adamantine/transparent to translucent |
| color | brown or black, sometimes reddish |
| streak | white |
Properties in Thin Section
Cassiterite is yellow, brown, red, or colorless in thin section. Relief and birefringence are high. Uniaxial (+), ω = 2.006, ε = 2.097, δ = 0.091.
Crystallography
Cassiterite is tetragonal, a = 4.74, c = 3.19, Z = 2; space group $ \small{P \frac{4_2}{m} \frac{2_1}{n} \frac{2}{m}} $; point group $ \small{ \frac{4}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Cassiterite crystals are pyramidal, stubby prismatic, or acicular. Faces may be striated. Contact and penetration twins are common; complex multiple twinning is less common. Cassiterite may be massive, colloform, reniform, or fibrous.
Structure and Composition
In cassiterite, distorted SnO6 octahedra share edges to form chains. Chains are connected by corner-sharing octahedra. Each O is in triangular coordination, bonded to three Sn. Cassiterite may contain minor Fe and Ta substituting for Sn. Mn, W, Nb, and Sc also may be present in trace amounts.
Occurrence and Associations
Cassiterite is widespread but rarely concentrated as tin ore. It occurs in pegmatites, veins, contact aureoles, altered zones of ore deposits, and placers. Associated minerals include quartz, topaz, tourmaline, fluorite, muscovite, lepidolite, wolframite, scheelite, and others.
Related Minerals
Cassiterite is isostructural with rutile, TiO2; pyrolusite, MnO2; plattnerite, PbO2; and paratellurite, TeO2.
▪Pyrolusite MnO2


Origin of Name
From the Greek words pyr and louein, meaning “fire” and “to wash,” referring to its use in glass manufacturing.
Hand Specimen Identification
Metallic luster, extreme softness (H = 1 to 2), sooty character, and a black streak characterize pyrolusite. Occurrence as arborescent dendrites (Figure 14.334), similar in appearance to snowflakes or frost on windows, is diagnostic. Massive samples, such as the one in Figure 14.335, may be difficult to distinguish from other dense black minerals.
Physical Properties
| hardness | 1 to 2 |
| specific gravity | 4.5 to 5.0 |
| cleavage/fracture | perfect but rarely seen prismatic {110}/uneven |
| luster/transparency | metallic/opaque |
| color | black |
| streak | black or bluish-black |
Crystallography
Pyrolusite is tetragonal, a = 4.40, c = 2.87, Z = 2; space group $ \small{P \frac{4_2}{m} \frac{2_1}{n} \frac{2}{m}} $; point group $ \small{ \frac{4}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Rare macroscopic crystals of pyrolusite are perfect tetragonal prisms. More commonly pyrolusite forms orthorhombic pseudomorphs after manganite, or it is dendritic, fibrous, reniform, or columnar.
Structure and Composition
Pyrolusite has the rutile structure (see rutile structure, above). It is commonly close to pure Mn-oxide, but Mn valence may be slightly variable. Small amounts of H2O may be present. Minerals with similar structures to rutile include cassiterite, SnO2; pyrolusite, MnO2; plattnerite, PbO2; and stishovite, SiO2.
Occurrence and Associations
Pyrolusite is a secondary mineral found as coatings, nodules, dendrites, and in beds. Associated minerals include barite, limonite, romanechite (psilomelane), hematite, magnetite, and other Mn- and Fe-oxides.
Varieties
Wad is the name for mixtures of Mn-oxides and hydroxides that include pyrolusite.
Related Minerals
Pyrolusite has several polymorphs, including nsutite, Mn(O,OH)2; ramsdellite, MnO2; and vernadite, (Mn,Fe,Ca,Na)(O,OH)2 · nH2O. Other related manganese minerals include manganite, MnO(OH); romanechite (psilomelane), BaMn9O16(OH)4; and birnessite, Na4Mn14O27•9H2O.
▪Columbite-Tantalite (Fe,Mn)(Nb,Ta)2O6
Origin of Name
Columbium, an early name for the element tantalum, is named after Columbia, where the original samples of columbite, (Fe,Mn)Nb2O6, were found. Tantalite, (Fe,Mn)Ta2O6, derives from the Greek myth of Tantalus. These two end members form a complete solid solution and are generally grouped together.

Hand Specimen Identification
Association, color, luster, streak, and density help identify members of the columbite–tantalite series, but distinguishing Nb-rich varieties from Ta-rich varieties is not possible without chemical analysis. Crystals often display heart-shaped twins – a hint of that shape is visible in Figure 14.336. These minerals may be confused with wolframite or uraninite, other dark-colored dense minerals.
Physical Properties
| hardness | 6 |
| specific gravity | 6.0 |
| cleavage/fracture | good (010)/subconchoidal |
| luster/transparency | submetallic/translucent to opaque |
| color | iron-black to brown |
| streak | brown, dark red to black |
Properties in Thin Section
Columbite-tantalite msinerals are biaxial (+), α = 2.44 , β = 2.32, γ = 2.38, δ = 0.12, 2V = 75°.
Crystallography
Columbite-tantalite minerals are orthorhombic, a = 5.10, b = 14.27, c = 5.74, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2}{c} \frac{2_1}{n}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Columbite-tantalite crystals are usually short prisms or tabs, sometimes with heart-shaped twins. Crystal aggregates and masses are common.
Structure and Composition
The structure of columbite-tantalite consists of chains of (Fe,Mn)O6 and (Nb,Ta)O6 octahedra. Edge sharing joins them together. Columbite, (Fe,Mn)Nb2O6, and tantalite, (Fe,Mn)Ta2O6 form a complete solid solution. Mg may substitute for (Fe,Mn). Sn and W may be present in small amounts.
Occurrence and Associations
Members of the columbite–tantalite series are uncommon. They occur in pegmatites with quartz, feldspar, mica, Li-minerals, phosphates, and other typical pegmatite minerals. They also occur in carbonatites and in placer deposits with other dense minerals.
Varieties
Tapiolite is a polymorph of columbite–tantalite. Other related minerals include microlite, Ca2Ta2O6(O,OH,F); pyrochlore, (Ca,Na)2(Nb,Ta)2O6(O,OH,F); and fergusonite, (REE)NbO4.
5.2 Spinels and other Oxides with Mixed Coordination
|
Inverse Spinels Normal Spinels Other Oxides |
In minerals of the spinel group, both tetrahedral and octahedral sites are occupied by metal ions. In what we call normal spinels, each metal species is found in either tetrahedral or octahedral coordination but not both. We can write the formula of normal spinels as XY2O4, with X representing the tetrahedral cation and Y the octahedral cation. In inverse spinels, one metal species occupies both coordinations. A general formula is Y[XY]O4, with the brackets identifying the two different metal ions in octahedral sites.
Mineralogists have identified other oxides that do not fit into the tetrahedral, octahedral, or spinel groups. In uraninite and thorianite, for example, metal atoms occupy cubic sites. In perovskite, Ti and Ca are in 6-fold and 12-fold coordinations, respectively. In cuprite, Cu is in 2-fold coordination; and chrysoberyl is isostructural with olivine.
For more general information about oxides, see Chapter 9: Ore Deposits and Economic Minerals.
▪Spinel MgAl2O4
Origin of Name
From the Latin word spina, meaning “thorn,” a reference to the sharp crystals.

Hand Specimen Identification
The term spinel is used in a generic sense to describe any of the many minerals with spinel structure. The term also refers to a specific mineral with composition MgAl2O4. This mineral is recognized by its octahedral crystals, hardness (H = 8), and vitreous luster. Spinel comes in many different colors, but the red color seen in Figure 14.337 is most common and most diagnostic.
Physical Properties
| hardness | 8 |
| specific gravity | 3.5 to 4.0 |
| cleavage/fracture | none/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | variable, red, lavender, blue, green, brown, white, or black |
| streak | white |
Properties in Thin Section
Nearly pure MgAl2O4 spinel is colorless in thin section but is pleochroic green or blue-green if Fe substitutes for Mg. Octahedral shape and high index of refraction aid identification. Isotropic, n = 1.74.
Crystallography
Spinel is cubic, a = 8.09, Z = 8; space group $ \small {F \frac{4}{d} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Spinel typically forms octahedral crystals; twinning and modifying faces are common. Massive forms and irregular grains are also known.
Structure and Composition
Spinel minerals have relatively simple cubic structures. MgO6 octahedra and AlO4 tetrahedra share edges and are closest packed. Fe, Zn, and Mn may substitute for Mg. Fe and Cr may substitute for Al.
Occurrence and Associations
Spinel is a high-temperature mineral found in metamorphosed carbonates or schists, as an accessory in mafic igneous rocks, and in placers. Associated minerals include calcite, dolomite, garnet, and Ca-Mg silicates in marbles; garnet, corundum, sillimanite, andalusite and cordierite in highly aluminous rocks; diopside, olivine, chondrodite, in mafic rocks; and other dense minerals in placers.
Varieties
Pleonaste is the name given to intermediate Fe-Mg spinels. Ruby spinel is the name of gemmy-red spinel; various other gem names are used to a lesser extent.
Related Minerals
Spinel is isostructural with other members of the spinel group and with bornhardite, Co3Se4; linnaeite, Co2S4; polydymite, Ni3S4; indite, FeIn2S4; and greigite, Fe3S4. Spinel forms solid solutions with other members of the spinel group, including hercynite, FeAl2O4; gahnite, ZnAl2O4; galaxite, MnAl2O4; zincochromite, ZnCr2O4; and magnesiochromite, MgCr2O4.
▪Magnetite Fe3O4
Origin of Name
Named after Magnesia, near Macedonia in Thessaly, where the Greeks found this mineral.


Hand Specimen Identification
Magnetite, a member of the spinel group, is similar to other dense, hard, dark-colored minerals. It is, however, characterized by its strong magnetism, which distinguishes it from ilmenite, chromite, and other similar looking minerals (Figure 14.338). Macroscopic euhedral crystals are uncommon, but form octahedra (Figure 14.339).
Physical Properties
| hardness | 6 |
| specific gravity | 5.20 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | metallic/opaque |
| color | black |
| streak | black |
Crystallography
Magnetite is a cubic mineral, a = 8.397, Z = 8; space group $ \small {F \frac{4}{d} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Magnetite crystals are octahedra that sometimes display contact or lamellar twins. Magnetite is also common as massive or granular aggregates or disseminated as fine grains.
Structure and Composition
Magnetite has the spinel structure (see spinel structure, above). Some Ti is usually present in magnetite; at high temperature a complete solid solution to Fe2TiO4 (ulvöspinel) is possible. Minor amounts of Mg, Mn, Ni, Al, Cr, and V may substitute for Fe.
Occurrence and Associations
Magnetite is common and widespread. It is found as an accessory in many types of igneous, metamorphic, and sedimentary rocks and in unconsolidated sediments. It may be concentrated to form ore bodies by magmatic, metamorphic, or sedimentary processes.
Related Minerals
Magnetite forms solid solutions with ulvöspinel, Fe2TiO4; magnesioferrite, MgFe2O4; jacobsite, MnFe2O4; and to a lesser extent with maghemite, Fe2O3.
▪Chromite FeCr2O4
Origin of Name
The name chromite refers to this mineral’s composition.


Hand Specimen Identification
Black color, high density, black/brown streak, metallic luster, and association distinguish chromite. It may be slightly magnetic and is sometimes confused with magnetite or ilmenite. The photos in these two photos show typical examples.
Physical Properties
| hardness | 5.5 |
| specific gravity | 4.5-4.8 |
| cleavage/fracture | none/conchoidal |
| luster/transparency | metallic/subtranslucent; opaque |
| color | black, brownish black |
| streak | brown, dark brown |
Crystallography
Chromite is a cubic mineral, a = 8.37, Z = 8; space group $ \small {F \frac{4}{d} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Rare euhedral crystals are octahedral; chromite is generally massive or granular.
Structure and Composition
The structure of chromite is the same as that of all the spinel minerals (see spinel structure). Mg, Fe, Al, and Zn are typical impurities.
Occurrence and Associations
Primary chromite is found with olivine, pyroxene, spinel, magnetite, and sulfides in ultramafic rocks. It is also found in placers and black sands. Chromite is commonly a minor accessory mineral but may be concentrated by gravity or magmatic processes.
Related Minerals
Chromite has one polymorph, donathite. Chromite forms solid solutions with magnesiochromite, MgCr2O4, and hercynite, FeAl2O4, and to lesser extent with other spinel minerals.
▪Franklinite ZnFe2O4
Origin of Name
Named after Franklin, New Jersey, the type locality where this mineral is found.

Hand Specimen Identification
Franklinite resembles other dark-colored minerals of the spinel group and some Fe-Ti oxides, but has a dark brown streak and is only slightly magnetic. Crystals are typically dark-colored octahedra (Figure 14.342). This mineral is found at, and around, Franklin, New Jersey, and nowhere else.
Physical Properties
| hardness | 6 |
| specific gravity | 5.32 |
| cleavage/fracture | none/conchoidal |
| luster/transparency | metallic/opaque |
| color | black or iron-black |
| streak | black, reddish brown to dark brown |
Crystallography
Franklinite is cubic, a = 8.43, Z = 8; space group $ \small {F \frac{4}{d} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Octahedral franklinite crystals, often with modifying faces, are common in Franklin, New Jersey, the only place where this mineral is found in large quantities. Franklinite also occurs as discrete rounded grains, as granular masses, or in massive lenses.
Structure and Composition
Franklinite‘s structure is the same as that of other spinel minerals (see spinel structure). Franklinite normally contains substantial Mn substituting for Zn. Mn may also substitute for Fe. Minor Mg, Cr, and V also may be present.
Occurrence and Associations
Franklinite is associated with zincite and willemite, two other zinc minerals, in zinc ore deposits at Franklin, New Jersey. The host rock is a coarse-grained limestone.
Related Minerals
Franklinite is similar in many ways to other dark-colored spinel minerals. It forms minor solid solutions with most of them.
▪Chrysoberyl BeAl2O4
Origin of Name
From the Greek words meaning “golden beryl.”


Hand Specimen Identification
Translucent to transparent character, light color, vitreous luster, extreme hardness (H = 8.5), and common twinning characterize chrysoberyl. Chrysoberyl looks superficially like any of a number of other light-colored translucent/transparent minerals, but is significantly harder than most. Greenish to yellow colors are typical, like the specimens in these two figures, but other hues are known.
When visible, chrysoberyl‘s orthorhombic symmetry helps identify chrysoberyl. Cyclic twinning, such as seen in Figure 14.344, is classic for this mineral.
Physical Properties
| hardness | 8.5 |
| specific gravity | 3.7 to 3.8 |
| cleavage/fracture | good but indistinct prismatic {011}, poor (010)/ subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | yellow, green, or brown |
| streak | white |
Properties in Thin Section
Chrysoberyl is biaxial (+), α = 1.747 , β = 1.748, γ = 1.757, δ = 0.010, 2V = 45°.
Crystallography
Chrysoberyl is orthorhombic, a = 4.24, b = 9.39, c = 5.47, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Chrysoberyl is generally tabular and sometimes heart shaped or pseudohexagonal due to cyclic twinning. Faces are often striated.
Structure and Composition
Chrysoberyl‘s structure, similar to that of olivine, contains hexagonal closest packed oxygens with Be in tetrahedral sites and Al in octahedral sites.
Occurrence and Associations
Chrysoberyl is a rare mineral occurring in granites, pegmatites, mica schists, and some placers.
Varieties
Cat’s eye (cymophane) is a green chatoyant gem variety of chrysoberyl. Alexandrite is an emerald-green gem variety that appears red under artificial light. Both are very valuable.
Related Minerals
Chrysoberyl is isostructural with olivine minerals. It is chemically similar to members of the spinel group but has a different structure.
▪Uraninite UO2
Origin of Name
The name uraninite refers to this mineral’s composition.



Hand Specimen Identification
Uraninite is characterized by its radioactivity, association with other radioactive minerals, high specific gravity, brown to black streak, black color, and luster. It can be confused with other dense, dark-colored minerals.
Figure 14.345 shows a typical nondescript sample of uraninite. In contrast, Figure 14.346 show a spectacular euhedral crystal cluster. Figure 14.347 is a photo of botryoidal uraninite. Each of the spheres grew outward from seeds at their centers and subsequently grew together to produce the cluster seen.
Physical Properties
| hardness | 5.5 |
| specific gravity | 7 to 9.5 |
| cleavage/fracture | none/conchoidal |
| luster/transparency | pitchy dull to submetallic/opaque |
| color | black |
| streak | brown to black |
Crystallography
Uraninite is cubic, a = 5.4682, Z = 4; space group $ \small {F \frac{4}{m} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Individual crystals of uraninite are rare but may form cubes, octahedra, or combinations. Massive, colloform, or botryoidal forms are more typical.
Structure and Composition
Uraninite is isostructural with fluorite (see fluorite structure, above). Uraninite is isostructural with thorianite, ThO2; cerianite, (Ce,Th)O2; and fluorite (CaF2). It is closely related to sellaite, MgF2, and frankdicksonite, BaF2.U valence and U:O ratios are somewhat variable; uraninite is really a mixture of UO2 and U3O8. Th may substitute for U; N, Ar, Fe, Ca, Zr, and rare earths are also commonly present. Pb and Ra are always present as radioactive decay products.
Occurrence and Associations
Uraninite occurs in granitic pegmatites, in veins, and in sandstones. Associated minerals include quartz, K-feldspar, zircon, tourmaline, and monazite in pegmatites; cassiterite, galena, sulfides, and arsenides in veins; and quartz and various other secondary minerals in sandstones. Uraninite also concentrates in coal and other organic debris in sediments and related sedimentary rocks.
Varieties
Massive forms of uraninite are called pitchblende.
Related Minerals
Uraninite is isostructural with fluorite, CaF2, and cerianite, (Ce,Th)O2. It forms complete solid solution with thorianite, ThO2. Other related minerals include baddeleyite, ZrO2.
▪Cuprite Cu2O
Origin of Name
From the Latin word cuprum, meaning “copper.”


Hand Specimen Identification
Red- or black-colored crystals, sometime showing internal reflection, octahedral crystal shape, adamantine luster, brownish red streak, and association all help identify cuprite. It may be confused with cassiterite, hematite, and cinnabar, all red minerals. It can sometimes also be confused with rutile and other red-black oxides but is denser than most of them. Figures 14.348 and 14.349 show two examples of cuprite crystals.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 6.14 |
| cleavage/fracture | imperfect {111}/conchoidal |
| luster/transparency | adamantine, submetallic or earthy/often translucent to transparent |
| color | dark red, sometimes almost black |
| streak | brownish red, metallic |
Crystallography
Cuprite is cubic, a = 4.27, Z = 2; space group $ \small {F \frac{4}{d} \overline{3}\ \frac{2}{m}} $; point group $ \small {\frac{4}{m} \overline{3}\frac{2}{m}}$.
Habit
Octahedral, cubic, and dodecahedral forms, often in combination, are common. Cuprite may occur as elongated capillary crystals called chalcotrichite.
Structure and Composition
Oxygen, in tetrahedral groups, is arranged in a body centered cubic array. Each Cu is bonded to two O. Cuprite is generally close to end-member composition; Fe is a common minor impurity.
Occurrence and Associations
Cuprite is a secondary mineral found in the oxidized zones of copper deposits. Native copper, limonite, and secondary copper minerals such as malachite, azurite, and chrysocolla are typically associated minerals.
Related Minerals
Tenorite, CuO, is similar in composition and occurrence.
6 Hydroxide Minerals
| VI. Hydroxides gibbsite Al(OH)3 brucite Mg(OH)2 manganite MnO(OH) goethite FeO(OH) diaspore AlO(OH) romanechite BaMn9O16(OH)4 |
The hydroxide minerals all contain OH– as an essential anion species. Some, such as diaspore also contain O2- as well. The atomic arrangements are generally simple; brucite and gibbsite have layered structures equivalent to the trioctahedral and dioctahedral layers in micas. Romanechite (also called psilomelane) has a structure related to rutile and spinel. Only limited solid solution occurs between the various hydroxides, but they are often found in intimate mixtures with each other and with oxide minerals.
Bauxite refers to a mixture of Al oxides and hydroxides, limonite to a mixture of Fe oxides and hydroxides, and wad to a mixture of Mn oxides and hydroxides.
For more general information about oxides, see Section 9.2.3 in Chapter 9.
▪Gibbsite Al(OH)3
Origin of Name
Named after Colonel G. S. Gibbs (1777–1834), a mineral collector.


Hand Specimen Identification
Earthy smell and appearance, and sometimes green or blue color help identify gibbsite. When clear, it looks like other soft clear minerals. It may occasionally be confused with kaolinite, talc, or brucite when uncolored. When light green, it looks like smithsonite.
Figures 14.350 and 14.351 show two samples of gibbsite from the same place in China – one green and one turquoise blue.
Physical Properties
| hardness | 2.5 to 3 |
| specific gravity | 2.40 |
| cleavage/fracture | perfect (001)/uneven |
| luster/transparency | vitreous, pearly, earthy/ transparent to translucent |
| color | greenish or aquamarine blue; less commonly white or gray |
| streak | white |
Properties in Thin Section
Gibbsite is usually colorless in thin section; maximum interference colors are upper first order. It is difficult to distinguish from clay minerals. Biaxial (+), α = 1.57 , β = 1.57, γ = 1.59, δ = 0.02, 2V = 0° to 40°
Crystallography
Gibbsite is monoclinic, a = 8.641, b = 5.07, c = 9.719, β = 94.57°, Z = 8; space group $ \small{P \frac{2_1}{n} } $; point group $ \small{\frac{2}{m} } $.
Habit
Gibbsite may form foliated or tabular crystals, typically very small with a pseudohexagonal shape. Granular aggregates, colloform or radiating masses, and coatings are most common.
Structure and Composition
In the layered gibbsite structure, octahedral Al3+ occupies two of the three sites between OH sheets. Small amounts of Fe may replace Al.
Occurrence and Associations
Gibbsite is a secondary mineral associated with aluminum deposits, bauxites, and laterites. Diaspore and böhmite, other aluminum hydroxides, are typically intimate associates.
Related Minerals
Gibbsite is similar in structure to brucite, Mg(OH)2. It has several polymorphs, including bayerite, doyleite, and nordstrandite. Other related minerals include diaspore and böhmite, both AlO(OH), and bauxite, a mixture of gibbsite, böhmite, and diaspore.
▪Brucite Mg(OH)2
Origin of Name
Archibald Bruce (1777–1818), an early American mineralogist, was the inspiration for this mineral’s name.


Hand Specimen Identification
Good platy or micaceous cleavage, flexible sheets, clear or light color, and luster help identify brucite. It may be confused with gypsum or gibbsite, other soft light minerals that may be clear. Color is variable, but translucent white, green, or blue crystals are most common. The two photos above show examples of brucite; Figure 8.76 shows another example.
Physical Properties
| hardness | 2.5 |
| specific gravity | 2.4 to 2.5 |
| cleavage/fracture | perfect basal (001)/sectile |
| luster/transparency | vitreous, pearly/transparent to translucent |
| color | white, light green or blue, or gray |
| streak | white |
Properties in Thin Section
Brucite is colorless in thin section and displays anomalous second-order red or blue interference colors. Brucite may be mistaken for talc, white mica, or gypsum, but they are all biaxial. Uniaxial (+), ω = 1.57, ε = 1.58, δ = 0.02.
Crystallography
Brucite belongs to the trigonal crystal system, a = 3.147, c = 4.769, Z = 1; space group $ \small {P\overline{3} \frac{2}{m}} $; point group $ \small {\overline{3}\frac{2}{m}}$.
Habit
Broad platy, foliated, or tabular crystals are typical for brucite. Massive or fibrous aggregates and foliated masses are common.
Structure and Composition
Brucite‘s structure is similar to that of gibbsite: sheets of OH sandwich Mg in octahedral coordination. Fe, Mn, and Zn may substitute in minor amounts for Mg.
Occurrence and Associations
Brucite occurs in veins of mafic rocks, serpentinite, or talc–chlorite schists, and in metamorphosed carbonates or marls. It also occurs as a secondary mineral that form from periclase. Associated minerals include chlorite and other secondary magnesium minerals in mafic rocks, and calcite, dolomite, talc, magnesite, and periclase in carbonates.
Related Minerals
Brucite is isostructural with gibbsite, Al(OH)3. It is isotypical with pyrochroite, Mn(OH)2; amakinite, (Fe,Mg)(OH)2; portlandite, Ca(OH)2; and theophrastite, Ni(OH)2.
▪Manganite MnO(OH)

Origin of Name
The name manganite refers to this mineral’s composition.
Hand Specimen Identification
High density, prismatic crystals that may be striated, and black color characterize manganite. It may be confused with pyrolusite, with which it is frequently found, but is significantly harder and has a brown streak.
| hardness | 4 |
| specific gravity | 4.2 to 4.4 |
| cleavage/fracture | perfect basal (010), good {110}/sectile |
| luster/transparency | submetallic/opaque |
| color | steel gray to black |
| streak | dark gray, black, sometimes red-brown |
Crystallography
Manganite is monoclinic, a = 8.84 , β = 5.23, c = 5.74, β = 90.0°, Z = 8; space group $ \small {B\frac{2_1}{d}\ } $; point group $ \small {\frac{2}{m}\ } $.
Habit
Manganite crystals are prismatic, sometimes twinned, and may show striations and complicated terminations. Bundled, stalactitic, columnar, bladed, or fibrous aggregates are common.
Structure and Composition
In manganite, O and OH are nearly hexagonal closest packed. Mn(O,OH)6 octahedra share corners to make a three-dimensional framework. Fe and Mg may replace Mn in small amounts.
Occurrence and Associations
Manganite is an uncommon secondary mineral found in veins with other Mn oxides or hydroxides, carbonate minerals, limonite, and barite.
Related Minerals
A number of manganese oxides and hydroxides are closely related, including pyrolusite, MnO2; partridgite and bixbyite, both Mn2O3; hausmannite, Mn3O4; hollandite, Ba2Mn8O16; romanechite, BaMn9O16(OH)4; pyrochroite, Mn(OH)2; vernadite, Mn(OH)4; and takanelite, (Mn,Ca)Mn4O9 · H2O. Wad is a mixture of these manganese minerals.
▪Goethite FeO(OH)
Origin of Name
Named after J. W. Goethe (1749–1832), a German poet and scientist.



Hand Specimen Identification
Brown color, sometimes earthy appearance, habit, and streak identify goethite. It is sometimes confused with hematite but has a brownish yellow streak, in contrast with hematite’s red streak. Most samples of goethite are fine-grained, rusty looking, and unexciting.
Figure 14.355 shows typical fine-grained earthy reddish goethite. In contrast, Figures 14.356 and 14.357 are photos of black euhedral goethite crystals. Goethite crystals such as these may be hard to distinguish from other black oxides and hydroxides unless orthorhombic symmetry is apparent. See also the photo of black goethite with crocoite in Figure 14.426.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 4.3 |
| cleavage/fracture | perfect but rarely seen (010)/uneven |
| luster/transparency | subadamantine to earthy/subtranslucent to opaque |
| color | yellow-brown to dark brown or black |
| streak | brown-yellow |
Properties in Thin Section
Goethite is pleochroic yellow, orange-red, or various shades of brown in thin section. It has high positive relief and extremely high birefringence that may be masked by its color. Goethite is difficult to distinguish from other Fe oxides. Biaxial (-), α = 2.26 to 2.26 , β = 2.39 to 2.41, = 2.40 to 2.52, δ = 0.15, 2V = 0° to 27°.
Crystallography
Goethite is orthorhombic, a = 4.65, b = 10.02, c = 3.04, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Euhedral goethite crystals are rare; it is usually platy, prismatic, fibrous, botryoidal, or mammillary. Concentric growth bands are common.
Structure and Composition
Goethite is isostructural with diaspore. Edge-sharing Fe(O,OH)6 octahedra form bands running parallel to the c-axis. Perpendicular to c, the bands form a checkerboard pattern, leaving empty channels between them. Up to several weight percent of Mn and absorbed water are often present.
Occurrence and Associations
Goethite is a widely distributed secondary mineral formed by the weathering of Fe-rich compounds. It concentrates in sediments, gossans, and laterites. Common associated minerals include siderite, pyrite, magnetite, and many residual weathering products.
Varieties
Bog ore is a porous, poorly consolidated form of goethite. Limonite refers to a mixture of hydrous iron oxides of variable chemistry and crystallinity.
Related Minerals
Lepidocrocite, akaganeite, and feroxyhyte are all rare polymorphs of goethite. Diaspore has the same structure except that Al replaces two-thirds of the Fe. Other related minerals are manganite, MnO(OH); heterogenite, CoO(OH); and montroseite, (V, Fe)O(OH).
▪Diaspore AlO(OH)
Origin of Name
From the Greek word diaspora, meaning “to scatter,” referring to its decrepitation when heated.


Hand Specimen Identification
Bladed habit, hardness, good cleavage, and luster identify diaspore. It is occasionally confused with brucite, but brucite is much softer.
Diaspore has one excellent cleavage, and Figure 14.358 shows typical cleavage fragments. The specimen in Figure 14.359 contains translucent gray diaspore with green chlorite and specks of magnetite from a classic collecting spot near Chester, Massachusetts.
Physical Properties
| hardness | 6.5 to 7 |
| specific gravity | 3.2 to 3.5 |
| cleavage/fracture | one perfect (010), poor (210)/conchoidal |
| luster/transparency | pearly, vitreous/translucent |
| color | colorless, yellow, gray, white or green |
| streak | white-yellow |
Properties in Thin Section
Diaspore has high relief, is colorless or pale, displays parallel extinction, and shows up to third-order interference colors. It may be confused with gibbsite or sillimanite, but gibbsite has lower relief and inclined extinction, and sillimanite has lower relief and birefringence. Biaxial (+), α = 1.68 to 1.71 , β = 1.71 to 1.72, γ = 1.73 to 1.75, δ = 0.04, 2V = 85°.
Crystallography
Diaspore is orthorhombic, a = 4.42, b = 9.40, c = 2.84, Z = 4; space group $ \small{P \frac{2_1}{b} \frac{2_1}{n} \frac{2_1}{m}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Platy, tabular, or acicular crystals of diaspore are common. Massive forms or foliated aggregates are also common.
Structure and Composition
Diaspore structure is the same as that of goethite, except that Al replaces two-thirds of the Fe (see goethite structure, above). Diaspore is generally close to end-member composition, but minor Fe or Mn may replace Al.
Occurrence and Associations
Diaspore is found in emery deposits with corundum, magnetite, spinel, and chlorite; in bauxites with other aluminum oxides and hydroxides; and as a rare mineral in some pegmatites.
Related Minerals
Böhmite is a polymorph of diaspore. Diaspore is isostructural with goethite and close in composition to gibbsite, Al(OH)3.
▪Romanechite (Psilomelane) BaMn9O16(OH)4
Origin of Name
From the original locality in Romaneche, France.

Hand Specimen Identification
Black color, submetallic to dull luster, and habit identify romanechite. Figure 14.360 shows the two most common habits for this mineral.
Romanechite is sometimes confused with pyrolusite, but pyrolusite is harder. A brown-black streak distinguishes romanechite from limonite and other hydrous iron oxides.
Physical Properties
| hardness | 5 to 6 |
| specific gravity | 3.5 to 4.7 |
| luster/transparency | submetallic, dull/opaque |
| color | black |
| streak | brown-black |
Crystallography
Romanechite is monoclinic, a = 9.56, b = 2.88, c = 13.85, β = 90.5°, Z = 2; space group $ \small{A\frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Romanechite is typically in reniform, botryoidal, or dendritic masses. Individual euhedral crystals are not known.
Structure and Composition
Romanechite‘s structure is related to the structures of rutile and spinel. Mn(O,OH)6 octahedra form distorted chains; Ba and H2O occupy holes between the chains. Many other elements, including As, V, W, Co, Cu, Ni, Mg, Ca, and alkalis may be present in small or trace amounts.
Occurrence and Associations
Romanechite is a rare secondary mineral associated with pyrolusite, manganite, calcite, and hematite.
Related Minerals
Related manganese minerals include pyrolusite, MnO2; manganite, MnO(OH); cryptomelane, KMn8O16; hollandite BaMn8O16; and wad, a mixture of manganese oxides and hydroxides.
7 Carbonates and Nitrates
Mineralogists divide carbonate minerals into three main groups based on atomic arrangement: the calcite group, dolomite group, and aragonite group. Several other species that have more complex structures and chemistries are classified separately.
Calcite group minerals have hexagonal structures related to the structures of halite and galena: six Ca, Mg, Fe, Mn, or Zn surround anionic (CO3)-2 groups and each metal atom is surrounded by six carbonate groups. Dolomite group minerals have structures similar to calcite’s, but Ca and Mg, Fe, or Mn occupy alternate layers. Aragonite group minerals are orthorhombic. Solid solutions are common within a structural group, although some carbonates, such as aragonite, are almost always close to end-member composition.
|
Calcite Group Dolomite Group |
Aragonite Group Other Carbonates |
Nitrate Group nitratite NaNO3 niter (saltpeter) KNO3 |
Due to very high solubility in water, nitrate minerals are rare. They have structures similar to carbonates, but contain monovalent rather than divalent cations because the (NO3)– anionic group is monovalent. Over half a dozen nitrates are known, but nitratite and niter are the only common ones in more than just a few localities.
For more general information about carbonates and nitrates, see Section 7.4.3 of Chapter 7.
7.1 Calcite Group Minerals
▪Calcite CaCO3
Origin of Name
From the Latin word calx, meaning “burnt lime.”


Hand Specimen Identification
Calcite is identified by its hardness of 3, rhombohedral cleavage, and effervescence in cold dilute HCl. It may be confused with dolomite or aragonite. Dolomite however, does not react as readily to HCl, and aragonite is orthorhombic. Calcite can also be distinguished from dolomite using a red alizarin stain that turns calcite pinkish.


Some calcite specimens contain euhedral crystals, but many are rhombohedral cleavage fragments like those in Figures 14.361 and 14.362. The photo in Figure 14.361 shows calcite with its most common whitish color. The photo in Figure 14.362 shows (less common) blue calcite cleavage fragments. Other colors too, are possible.
When euhedral, calcite may form crystal clusters such as those seen in Figures 14.363 and 14.364. Note that the crystals in these two photos have significantly different shapes. Calcite commonly twins; Figure 7.40 (Chapter 7) shows one example.
Physical Properties
| hardness | 3 |
| specific gravity | 2.71 |
| cleavage/fracture | perfect rhombohedral {101}/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless to white; may also be tinted many other light colors when impure |
| streak | white |
Properties in Thin Section
Calcite is colorless in thin section and has extremely high birefringence, resulting in pale, washed out, or “pearl” white interference colors. Polysynthetic twinning is nearly always visible. This mineral shows high variable relief upon stage rotation. In thin section, calcite may be confused with other hexagonal carbonates. Orthorhombic carbonates, however, have parallel extinction and are biaxial. Calcite is uniaxial (-), ω = 1.658, ε = 1.486, δ = 0.172.
Crystallography
Calcite forms trigonal crystals. a = 4.99, c = 17.04, Z = 6; space group $ \small {R\overline{3}\frac{2}{c}}$; point group $ \small {\overline{3}\frac{2}{m}}$.
Habit
Calcite has many habits. Coarse crystals are highly variable. The most common forms are hexagonal prisms with simple to complex terminations, scalenohedra (often with combinations of other forms), rhombohedra (either acute or flattened), and tabs with well-developed basal faces. Polysynthetic twinning is common but usually requires a microscope to detect. Besides forming coarse crystals, calcite may be a fine-grained massive rock-forming mineral, it may make up fine to coarse granular aggregates, and it may precipitate as nodules or crusts or in speleothems.
Structure and Composition
Calcite is isostructural with other members of the calcite group. In calcite, Ca2+ ions alternate with (CO3)2- groups in a three-dimensional array. The atomic arrangement is related to the structure of cubic salts, such as halite or periclase, but is not cubic because the structure has been squashed along the equivalent of a main diagonal of the cube. The shortened direction is the c-axis in calcite; planar (CO3)2- groups are perpendicular to c, giving the structure a 3-fold axis of symmetry in that direction only. Mg, Fe, Mn, Zn, and a number of others may substitute for some of the Ca; except for Mn, most solid solutions are quite limited.
Occurrence and Associations
Calcite is a common and widespread mineral. It is an essential and major mineral in limestones and marbles, occurs in cave deposits, and occurs as a vein mineral with other carbonates, sulfides, barite, fluorite, and quartz. Calcite also occurs in some rare carbonate-rich igneous rocks and is a common cement in some sandstones. Calcite is an accessory mineral in rocks of many sorts. It is also a common weathering product. Organic calcite is found in shells and skeletal material.

Varieties
Several different varieties of calcite, having special properties, have their own name. Iceland spar, for example, is the name given to clear calcite, usually in rhombohedral cleavage fragments. Iceland spar is one of a very few mineral varieties that cause double refraction that can be seen without the aid of a petrographic microscope. An image behind a crystal appears double when viewed through the crystal. Figure 14.365 is a photo of Iceland spar that displays this property.


Mineralogists use the name dogtooth spar to describe calcite crystals with steep scalenohedral forms, such as the orangish calcite crystal in Figure 14.366. The name nail-head spar is used for crystals composed of flat rhombs or stubby prismatic crystals like those seen in Figure 14.367.
Related Minerals
Calcite has two polymorphs, aragonite, and vaterite. It is isostructural with magnesite, MgCO3; siderite, FeCO3; sphaerocobaltite, CoCO3; smithsonite, ZnCO3; nitratite, NaNO3; dolomite, CaMg(CO3)2; and gaspeite, (Ni,Mg,Fe)(CO3). Calcite and rhodochrosite form extensive solid solutions at room temperature and a complete solid solution above about 550 ºC (1,020ºF). Calcite forms limited solid solutions with ankerite, CaFe(CO3)2; dolomite, CaMg(CO3)2; and kutnohorite, CaMn(CO3)2, at all temperatures.
▪Magnesite MgCO3
Origin of Name
The name refers to its composition.


Hand Specimen Identification
Massive forms of magnesite may be chalky or porcelain-like, such as the specimens seen in Figure 14.368. When massive and fine-grained like the samples shown in this photo, mineral identification may be uncertain.
Magnesite may be confused with other soft white minerals, especially other carbonates. It is occasionally confused with chert but has inferior hardness. Coarse crystals of magnesite are rhomb-shaped and display rhombohedral cleavage (Figure 14.369) – identifying it as a carbonate – but it may be difficult to distinguish from other rhombohedral carbonates. However, magnesite is denser than dolomite and does not react to cold HCl like calcite, which helps with identification.
Physical Properties
| hardness | 3.5 to 5 |
| specific gravity | 3.00 |
| cleavage/fracture | perfect rhombohedral {101}/conchoidal |
| luster/transparency | porcelainous/transparent to translucent |
| color | white, gray, brown, or yellow |
| streak | white |
Properties in Thin Section
Magnesite is similar to calcite in thin section but has higher index of refraction (see calcite optics). Uniaxial (-), ω = 1.700, ε = 1.509, δ = 0.191.
Crystallography
Magnesite forms trigonal crystals. a = 4.59, c = 14.87, Z = 6; space group $ \small {R\overline{3}\frac{2}{c}}$; point group $ \small {\overline{3}\frac{2}{m}}$.
Habit
Coarse crystals are rare; magnesite is usually massive, granular, fibrous, or earthy.
Structure and Composition
Magnesite is isostructural with calcite and other members of the calcite group (see calcite structure, above). Large amounts of Fe commonly substitute for Mg. Mn, Ca, Ni, and Zn may also be present in small amounts.
Occurrence and Associations
Magnesite is most common in veins or masses as an alteration product of mafic minerals. It also occurs in some Mg-rich schists and as a primary mineral in some rare chemical sediments. It is sometimes found as a replacement for calcite or dolomite in limestone.
Varieties
Breunnerite is a Fe-rich variety of magnesite; hoshiite is an Ni-rich variety.
Related Minerals
Magnesite forms complete solid solutions with siderite, FeCO3, and with gaspeite, (Ni,Mg,Fe)CO3. Other related minerals include hydromagnesite, Mg5(CO3)4(OH)2•4H2O.
▪Siderite FeCO3
Origin of Name
From the Greek word sideros, meaning “iron.”

Hand Specimen Identification
Coarse siderite crystals have the same rhombohedral shape and cleavage as other rhombohedral carbonates (Figure 14.370). Siderite can be distinguished from the other carbonates by its high specific gravity and brownish-green color. It effervesces slightly in warm HCl. It may be confused with sphalerite (which can have a similar brown color and the same hardness).
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 3.96 |
| cleavage/fracture | perfect rhombohedral {101}/subconchoidal |
| luster/transparency | vitreous/translucent |
| color | various shades of brown or brown-green are typical |
| streak | white |
Properties in Thin Section
Siderite is colorless to pale yellow-brown in thin section. It appears similar to calcite and other carbonates, but the other carbonates have a lower index of refraction and lack color. Uniaxial (-), ω = 1.875, ε = 1.633, δ = 0.242.
Crystallography
Siderite forms trigonal crystals. a = 4.72, c = 15.46, Z = 6; space group $ \small {R\overline{3}\frac{2}{c}}$; point group $ \small {\overline{3}\frac{2}{m}}$.

Habit
Siderite crystals are typically rhombohedra, often with curved faces. Fine- to coarse-grained aggregates, such as the reticulated aggregate seen in Figure 14.371, are common. Aggregates may also be colloform, globular, botryoidal, fibrous, or earthy.
Structure and Composition
Siderite is isostructural with calcite and other members of the calcite group (see calcite structure). Mn and Mg often substitute for Fe. Small amounts of Ca, Zn, and Co may be present.
Occurrence and Associations
Siderite is a relatively common mineral found in veins with galena, pyrite, chalcopyrite, and tetrahedrite; as a rock-forming mineral associated with limestone, clay, shale, coal or ironstone; as a replacement mineral in limestone; and less commonly in metamorphic rocks.
Related Minerals
Siderite is isostructural with calcite and a number of other minerals (see calcite related minerals, above). It forms complete solid solutions with rhodochrosite, MnCO3, and magnesite, MgCO3.
▪Rhodochrosite MnCO3
Origin of Name
From the Greek words meaning “rose” and “color,” referring to its rose-pink color.


Hand Specimen Identification
Pink color and rhombohedral carbonate morphology identify rhodochrosite. The two photos here show examples from Silverton, Colorado. Some rhodochrosite specimens have a light-pink to beige color, like the one in Figure 14.372. Others may be deeply colored, like the rhombs in Figure 14.373. Figure 7.30 is a photo of pink rhodochrosite rhombs with quartz.
Its rhomb-shaped crystals help identify this mineral but, if crystal morphology is uncertain, rhodochrosite may be mistaken for rhodonite (also a pink Mn-mineral). Rhodonite, however, is significantly harder (H = 5.5 to 6, compared with rhodochrosite’s 3.5 to 4). Rhodochrosite also effervesces slightly with cold dilute hydrochloric acid; rhodonite does not effervesce at all.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 3.70 |
| cleavage/fracture | perfect rhombohedral {101}/uneven |
| luster/transparency | vitreous, pearly/transparent to translucent |
| color | rose-red, light pink to dark brown |
| streak | white |
Properties in Thin Section
Rhodochrosite is colorless or pale pink in thin section, has extremely high birefringence, and three perfect cleavage directions. It may be confused with calcite and other carbonates. Uniaxial (-), ω = 1.816, ε = 1.597, δ = 0.219.
Crystallography
Rhodochrosite belongs to the trigonal system. a = 4.74, c = 15.51, Z = 6; space group $ \small {R\overline{3}\frac{2}{m}}$; point group $ \small {\overline{3}\frac{2}{m}}$.
Habit
Rhodochrosite forms rare rhombohedral crystals. It is usually fine-grained and massive, sometimes granular, botryoidal, columnar, or crusty.
Structure and Composition
Rhodochrosite is isostructural with calcite and other members of the calcite group (see calcite structure, above). Zn commonly replaces some Mn; Ca, Mg, Cd, and Co may be present in limited amounts.
Occurrence and Associations
Rhodochrosite is uncommon. It is found with other manganese minerals in Mn-rich metamorphic rocks, as a primary mineral in sulfide veins and some replacement bodies, and as a secondary mineral in residual deposits.
Related Minerals
Rhodochrosite forms solid solutions with calcite, CaCO3; siderite, FeCO3; and kutnohorite, CaMn(CO3)2.
▪Smithsonite ZnCO3
Origin of Name
Named after James Smithson (1754–1829), founder of the Smithsonian Institute.



Hand Specimen Identification
Rhombohedral carbonate habit, color (typically light green or purplish), high density, and association with other Zn-minerals identify smithsonite. If not distinctly colored, it may be difficult to tell from other dense carbonates. It is occasionally confused with hemimorphite, another commonly light-green soft mineral. The light-green and lilac colors seen in the first two photos above are typical; the stronger green-turquoise color in Figure 14.376 is less common.
Physical Properties
| hardness | 4 to 4.5 |
| specific gravity | 4.43 |
| cleavage/fracture | perfect rhombohedral {101}/subconchoidal |
| luster/transparency | pearly, vitreous/transparent to translucent |
| color | typically green; also purple, lilac, pink, and other colors |
| streak | white |
Properties in Thin Section
Smithsonite is uniaxial (-), ω = 1.850, ε = 1.625, δ = 0.225.
Crystallography
Smithsonite belongs to the trigonal crystal system. a = 4.61, c = 14.88, Z = 6; space group $ \small {R\overline{3}\frac{2}{m}}$; point group $ \small {\overline{3}\frac{2}{m}}$.
Habit
Crystals, when they are euhedral or subhedral, show rhombohedral form and cleavage. More typical smithsonite is massive, colloform, earthy, stalactitic, or forms crusts.
Structure and Composition
Smithsonite is isostructural with calcite and other members of the calcite group (see calcite structure, above). It typically contains substantial amounts of Fe. It may also contain smaller amounts of Ca, Co, Cu, Cd, Mg, or Mn, and traces of Ge or Pb.
Occurrence and Associations
Smithsonite is a secondary mineral found in zinc deposits. Associated minerals include sphalerite, hemimorphite, cerussite, malachite, azurite, and anglesite.
Related Minerals
Smithsonite forms limited solid solutions with most other carbonates, including otavite, CdCO3.
7.2 Dolomite Group Minerals
▪Dolomite CaMg(CO3)2
Origin of Name
Named after Déodat de Dolomieu (1750–1801), a French chemist and geologist.



Hand Specimen Identification
Dolomite is characterized by typical rhombohedral carbonate habit and cleavage. It effervesces slightly by reaction with cold dilute HCl when powdered, but not when solid.
The light pink/beige color of the reticulated dolomite in Figures 14.377 and 14.378 sometimes helps identify this mineral, but color is not a particularly reliable property and dolomite can be white or other colors. When white (Figure 14.379) it may be mistaken for calcite. When brown or tan, it may be mistaken for ankerite.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 2.85 |
| cleavage/fracture | perfect rhombohedral {101}/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | usually a shade of pink, can be white, colorless, brown, black, green, or gray |
| streak | white |
Properties in Thin Section
Dolomite is similar to calcite in thin section, but has lower RI and two possible orientations of polysynthetic twins. Uniaxial (-), ω = 1.679, ε = 1.500, δ = 0.179.
Crystallography
Dolomite is hexagonal (rhombohedral), a = 4.84, c = 15.96, Z = 3; space group $ \small{R3}$; point group $ \small{3}$.
Habit
Dolomite forms rhombohedral crystals, having the shape of cleavage fragments, often with curved faces. Less commonly they are prismatic or steep rhombohedra. Lamellar twinning is nearly always present but may be hard to see. Massive subhedral or anhedral dolomite, showing rhombohedral cleavage, is common.
Structure and Composition
Dolomite is isostructural with calcite, CaCO3, nordenskiöldine, CaSnB2O6, and with a number of other minerals (see calcite structure). Fe and Mn may substitute for Mg in substantial amounts. Co, Pb, Zn, Ce, or excess Ca may also be present.
Occurrence and Associations
Dolomite is a common mineral, found in massive carbonate sediments and in marbles, often with calcite. It also occurs in hydrothermal veins with fluorite, barite, other carbonates, and quartz, and as a secondary mineral or alteration product in limestone.
Related Minerals
Huntite, CaMg3(CO3)4, has quite similar properties. Dolomite forms solid solutions with ankerite (Ca-Fe carbonate), kutnohorite (Ca-Mn carbonate), minrecordite (Ca-Zn carbonate), and norsethite (Ba-Mg carbonate).
▪Ankerite CaFe(CO3)2
Origin of Name
Named after M. J. Anker (1772–1843), an Austrian mineralogist.

Hand Specimen Identification
Typical rhombohedral carbonate habit and cleavage, effervescence by cold dilute HCl when powdered, and yellow-brown to brown color usually identify ankerite. It is sometimes confused with calcite or dolomite, and less commonly with siderite. This photo shows crystals of ankerite on quartz.
Physical Properties
| hardness | 3.5 |
| specific gravity | 3.10 |
| cleavage/fracture | perfect rhombohedral {101}/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | white, yellow-brown |
| streak | white |
Properties in Thin Section
Ankerite is similar to dolomite in thin section, but tends to be stained red or brown by iron oxidation. Uniaxial (-), ω = 1.750, ε = 1.548, δ = 0.202.
Crystallography
Ankerite is a trigonal mineral. a = 4.82, c = 16.14, Z = 3; space group $ \small{R3}$; point group $ \small{3}$.
Habit
Rare crystals of ankerite are usually rhombs often with curved faces, or prisms. Lamellar twinning is nearly always present but may be hard to see. Granular ankerite is sometimes found. Massive subhedral to anhedral ankerite showing rhombohedral cleavage is most typical.
Structure and Composition
Ankerite is isostructural with calcite, dolomite, and many other minerals (see calcite structure). It may contain substantial Mg replacing Fe. Minor amounts of Mn, Co, Pb, Zn, Ce, or excess Ca may also be present.
Occurrence and Associations
Ankerite is most common in Precambrian iron formations. It is also found in veins and as replacements in limestones.
7.3 Aragonite Group Minerals
▪Aragonite CaCO3
Origin of Name
Named after the original locality in Aragon, Spain.



Hand Specimen Identification
Aragonite’s softness (H = 3.5 to 4), color, and association help identify it. Like calcite, it effervesces in cold dilute HCl. However, it does not have well-developed rhombohedral cleavage like calcite. If not well crystallized or showing orthorhombic cleavage, it may be difficult to distinguish from calcite. Aragonite, if white or clear, is also sometimes confused with strontianite.
Figure 14.381 shows a classic radiating cluster of orangish aragonite crystals (orthorhombic prisms) from Morocco. The color and morphology of this sample are diagnostic for aragonite. Figure 11.12 (Chapter 11) shows a specimen with crystals of similar morphology; the specimen is from Spain.
Figure 14.382 is a photo of white aragonite crystals with green malachite (a hydrated copper carbonate). When white, distinguishing aragonite from other white carbonates may be difficult. Figure 14.383 is a photo of helictites, a kind of cave formation made of aragonite; helictites form when CaCO3 precipitates from water.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 2.94 |
| cleavage/fracture | good (010), poor {110}/ subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless to white, and pale yellow or tan |
| streak | white |
Properties in Thin Section
Aragonite is similar to calcite in thin section but, because it is orthorhombic, displays parallel extinction. It is biaxial (-) with a small 2V. a = 1.530 , β = 1.681, γ = 1.685, δ = 0.155, 2V = 18°.
Crystallography
Aragonite is orthorhombic, a = 4.95, b = 7.96, c = 5.73, Z = 4; space group $ \small{P \frac{2_1}{m} \frac{2_1}{c} \frac{2_1}{n}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Aragonite crystals are commonly acicular, tabular, prismatic, or fibrous. Crystals may form radiating sprays, crusts, or masses of many different morphologies. Contact and cyclic twins are common, sometimes giving it a pseudohexagonal appearance.
Structure and Composition
In aragonite, triangular (CO3)2- groups are in layers perpendicular to the c-axis, as in calcite. Alternate layers, however, have their (CO3)2- groups pointing in opposite directions. Ca is coordinated to nine oxygens in six surrounding (CO3)2- groups, giving orthorhombic (pseudohexagonal) symmetry. Solid solutions are much more restricted than for calcite. Aragonite is usually near end-member CaCO3, with only minor amounts of Sr, Pb, or Zn substituting for Ca.
Occurrence and Associations
Aragonite is found as disseminated carbonate in gypsum beds, as hot spring deposits, as precipitates from Ca-oversaturated waters, associated with sedimentary iron ores, in oxidized zones of ore deposits, in some cave formations, and in blueschist facies metamorphic rocks. It also occurs in shells and other organic carbonate material. Associated minerals typically include gypsum, siderite, celestite, sulfur, limonite, calcite, malachite, azurite, smithsonite, and cerussite.

Varieties
Flos ferri (Figure 14.384) is a coral-like form of aragonite associated with iron deposits.
Related Minerals
Aragonite has two significant polymorphs, calcite and vaterite. Strontianite, SrCO3; witherite, BaCO3; cerussite, PbCO3; and niter, KNO3, are all isostructural with aragonite.
▪Witherite BaCO3
Origin of Name
Named after D. W. Withering (1741–1799), who first demonstrated that witherite was different from barite.

Hand Specimen Identification
Clear or lightly colored translucent orthorhombic crystals, relatively high density (easily discerned by the heft test), effervescence with dilute HCl, and hardness of 3-3.5 identify witherite. It is occasionally confused with barite, but barite does not react with HCl. Figure 14.385 shows an example of witherite from Cumbria, England.
Physical Properties
| hardness | 3.5 |
| specific gravity | 4.29 |
| cleavage/fracture | good basal (010), poor {110} and {012}/uneven |
| luster/transparency | resinous/transparent to translucent |
| color | gray, white, or colorless |
| streak | white |
Properties in Thin Section
Witherite is similar to aragonite and other orthorhombic carbonates in thin section. It may be confused with strontianite, but the latter has lower RI and two good cleavages. Biaxial (-), α = 1.529 , β = 1.676, γ = 1.677, δ = 0.148, 2V = 16°.
Crystallography
Witherite is orthorhombic, a = 5.26, b = 8.85, c = 6.55, Z = 4; space group $ \small{P \frac{2_1}{m} \frac{2_1}{c} \frac{2_1}{n}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Witherite crystals are orthorhombic, but multiple twinning yields pseudohexagonal pyramids. Columnar, globular, and botryoidal aggregates are common.
Structure and Composition
The structure of witherite is the same as that of aragonite. Minor Sr, Mg, and Ca may substitute for Ba.
Occurrence and Associations
Witherite is a rare low-temperature vein mineral, usually associated with galena and barite.
Related Minerals
Witherite has two high-temperature polymorphs. Strontianite, SrCO3; aragonite, CaCO3; cerussite, PbCO3; and niter, KNO3, are all isostructural with witherite.
▪Strontianite SrCO3
Origin of Name
Named after the first known locality at Strontian, Scotland.


Hand Specimen Identification
Strontianite is generally colorless or lightly colored, vitreous, and translucent. The photos show two examples of this mineral. Strontianite forms orthorhombic crystals, has moderate density, and reacts with cold dilute HCl. When amorphous, and the orthorhombic nature of crystals cannot be seen, it is difficult to distinguish strontianite from other white minerals with moderate density and hardness. It may be confused with aragonite but has different cleavage.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 3.72 |
| cleavage/fracture | good prismatic {110}, poor (010)/uneven |
| luster/transparency | vitreous/transparent to translucent |
| color | white, pale green, yellow, gray |
| streak | white |
Properties in Thin Section
Strontianite is similar to aragonite and other orthorhombic carbonates in thin section. Aragonite and witherite both have higher indices of refraction, aragonite has only one well-developed cleavage, and witherite has a larger 2V. Biaxial (-), a = 1.520 , β = 1.667, γ = 1.668, δ = 0.148, 2V = 7°.
Crystallography
Strontianite is orthorhombic, a = 5.13, b = 8.42, c = 6.09, Z = 4; space group $ \small{P \frac{2_1}{m} \frac{2_1}{c} \frac{2_1}{n}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Strontianite is typically acicular but may be prismatic, fibrous, granular, or massive. Twins, creating a pseudohexagonal or lamellar appearance, are common.
Structure and Composition
Strontianite has one high-temperature polymorph. Aragonite, CaCO3; witherite, BaCO3; cerussite, PbCO3; and niter, KNO3, are all isostructural with strontianite. Substantial replacement of Sr by Ca or Ba is common; Pb may also be present.
Occurrence and Associations
Strontianite is an uncommon mineral. It occurs in hydrothermal veins with barite, celestite, and calcite. Hosts include limestones, sulfide veins, vugs, and concretions.
Related Minerals
Strontianite is one of the two important Sr-minerals; the other is celestite (Sr-sulfate).
▪Cerussite PbCO3
Origin of Name
From the Latin word cerussa, meaning “white lead.”


Hand Specimen Identification
High density (easily discerned by the heft test), color, and luster identify cerussite. It may be confused with anglesite (Pb-sulfate) but it has a different habit. Additionally, cerussite effervesces in cold dilute HCl; anglesite does not.
Figure 14.388 is a typical reticulated cluster of cerussite crystals. Cerussite is one of a small number of minerals that commonly display cyclic twins; Figure 14.389 shows an example from Namibia.
Physical Properties
| hardness | 3 – 3.5 |
| specific gravity | 6.5-6.6 |
| cleavage/fracture | good prismatic {110}, distinct {021}/conchoidal |
| luster/transparency | adamantine, vitreous, resinous, pearly, dull, earthy/translucent |
| color | colorless, white to light tan, less commonly gray, blue, or green |
| streak | white |
Properties in Thin Section
Cerussite is biaxial (-), a = 1.804 , β = 2.076, γ = 2.078, δ = 0.274, 2V = 9°.
Crystallography
Cerussite is orthorhombic, a = 5.15, b = 8.47, c = 6.11, Z = 4; space group $ \small{P \frac{2_1}{m} \frac{2_1}{c} \frac{2_1}{n}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Cerussite crystals are variable but most commonly tabular. Twinning gives a pseudohexagonal appearance. Cerussite may also appear prismatic, acicular, granular, and massive. Coarse intergrowths and clusters with platy or reticulated fabric are typical.
Structure and Composition
Strontianite, SrCO3; aragonite, CaCO3; witherite, BaCO3; and niter, KNO3, are all isostructural with cerussite (see aragonite structure). It is generally quite close to end-member composition, although minor amounts of Ba, Sr, Ag, or Zn are sometimes present.
Occurrence and Associations
Cerussite is a common secondary lead mineral found in altered ore deposits. Typical associated minerals include galena, anglesite, limonite, and pyromorphite. It occurs in both veins and bedded deposits.
Related Minerals
Hydrocerussite, Pb3(CO3)2(OH)2, is a closely related mineral.
7.4 Malchite and Azurite
▪Malachite Cu2CO3(OH)2
Origin of Name
From the Greek word moloche, meaning “mallows,” referring to the green color of mallow leaves.
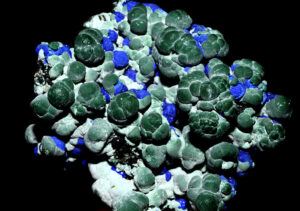

Hand Specimen Identification
Distinctive light to dark green color, occurrence as a secondary mineral that often replaces or coats other minerals, habit, and association with other copper minerals (especially azurite) help identify malachite. It may be confused with other secondary copper minerals but effervesces in cold dilute HCl, unlike most of the others.
Figure 14.390 shows typical green malachite. It has crystallized on top of a rusty mix of iron oxides/hydroxides, forming as an alteration product of other (primary) Cu- and Fe-minerals. Malachite and azurite, both hydrated copper carbonates, commonly occur together. Figure 14.391 shows a specimen from Morocco that contains both minerals. Figure 1.4 is a photo of another sample that contains malachite and azurite. Figure 14.348, above, shows malachite with cuprite (Cu-oxide).
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 3.7 to 4.0 |
| cleavage/fracture | perfect (201)/subconchoidal |
| luster/transparency | adamantine/transparent to translucent |
| color | bright green |
| streak | pale green |
Properties in Thin Section
Malachite is biaxial (-), a = 1.655 , β = 1.875, γ = 1.909, δ = 0.254, 2V = 43°.
Crystallography
Malachite is monoclinic, a = 9.48, b = 12.03, c = 3.21, β = 98.0°, Z = 4; space group $ \small{P \frac{2_1}{a}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Coarse malachite crystals are rare. Malachite is usually fine-grained and massive, frequently colloform or banded, and often intergrown with other secondary copper minerals.
Structure and Composition
Malachite‘s structure resembles those of other carbonates. Triangular (CO3)2- groups are surrounded by three Cu(O,OH)6 octahedra. The octahedra share edges to form chains. Zn and Co are commonly present in small amounts.
Occurrence and Associations
Malachite is a secondary copper mineral typically found in carbonate rocks with azurite, cuprite, native copper, limonite, and chrysocolla.
Related Minerals
Similar minerals include other hydrated Cu and Zn carbonates, including azurite, Cu3(CO3)2(OH)2; hydrozincite, Zn5(CO3)2(OH)6; aurichalcite, (Zn,Cu)5(CO3)2(OH)6; and a number of other rare hydrated Cu and Zn carbonates.
▪Azurite Cu3(CO3)2(OH)
Origin of Name
From the French word azur, meaning “sky-blue color.”


Hand Specimen Identification
Azurite’s softness, characteristic blue color, association with malachite, effervescence in cold HCl, and habit are distinctive.
Figure 14.392 is a photo of spectacular blue azurite, but samples like this are uncommon. The photo in Figure 14.393 shows a more typical example. The azurite is not as blue as in the previous photo, and the crystals are not as coarse. This specimen also contains minor amounts of green malachite. The azurite and malachite are on top of rusty iron oxides/hydroxides.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 3.77 |
| cleavage/fracture | brittle, perfect {011}, good {100}/conchoidal |
| luster/transparency | normally dull and earthy/transparent to translucent |
| color | blue |
| streak | blue |
Properties in Thin Section
Azurite is biaxial (+), a = 1.730 , β = 1.756, γ = 1.836, δ = 0.106, 2V = 68°.
Crystallography
Azurite is monoclinic, a = 4.97, b = 5.84, c = 10.29, β = 92.4°, Z = 2; space group $ \small{P \frac{2_1}{c}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Azurite is typically massive and earthy. It may form as a crust on other copper minerals. Rarer individual crystals are tabular or prismatic.
Structure and Composition
Usually nearly pure copper carbonate, azurite has a complex structure consisting of Cu2+ ions coordinated to two oxygens of adjacent (CO3)2- groups and to two OH– groups, making a square planar arrangement. The square groups are linked to form chains.
Occurrence and Associations
Azurite, like malachite, is a secondary copper mineral formed by alteration of copper oxides and sulfides. It is less common than malachite.
Related Minerals
Chemically similar minerals include malachite, Cu2CO3(OH)2; hydrocerussite, Pb3(CO3)2(OH)2; hydromagnesite, Mg5(CO3)4(OH)2; and aurichalcite, (Zn,Cu)5(CO3)2(OH)3.
7.5 Nitrate Group Minerals
▪Nitratite (Soda Niter) NaNO3
Origin of Name
Nitratite is one of the two more common nitrate minerals.

Hand Specimen Identification
Softness, distinctive “cooling” taste, habit, and solubility help identify nitratite. It is typically found in thin layers or coatings deposited by efflorescence in dry environments. It is deliquescent (absorbs water and becomes liquid) under humid conditions.
Physical Properties
| hardness | 1 to 2 |
| specific gravity | 2.29 |
| cleavage/fracture | perfect but rarely seen rhombohedral {104}/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless |
| streak | white |
Properties in Thin Section
Nitratite is uniaxial (-), ω = 1.587, ε = 1.336, δ = 0.251.
Crystallography
Nitratite is a trigonal mineral. a = 5.07, c = 16.82, Z = 6; space group $ \small {R\overline{3}\frac{2}{c}}$; point group $ \small {\overline{3}\frac{2}{m}}$.
Habit
Individual nitratite crystals have a rhombohedral habit, but nitratite is often too fine grained or too massive for crystals to be easily seen. It sometimes forms as a crust.
Structure and Composition
Nitratite, the sodium analogue of niter (saltpeter; KNO3), is isostructural with calcite. Na and NO3 replace for Ca2+ and CO, respectively in calcite. Nitratite is always nearly pure, forming very limited solid solution with niter (saltpeter).
Occurrence and Associations
Because nitratite is highly soluble in water, it is only found in arid regions where it may be associated with other evaporite minerals.
Related Minerals
The only other common nitrate is niter (saltpeter).
▪Niter (Saltpeter) KNO3
Origin of Name
Niter is the second most common nitrate mineral, and is quite rare.

Hand Specimen Identification
Softness, salty taste, habit, and high solubility in water help identify this mineral.
Physical Properties
| hardness | 2 |
| specific gravity | 2.10 |
| cleavage/fracture | perfect but rarely seen rhombohedral {011}/uneven |
| luster/transparency | vitreous/translucent |
| color | white |
| streak | white |
Properties in Thin Section
Niter is biaxial (-), a = 1.333 , β = 1.505, γ = 1.505, δ = 0.172, 2V = 7°.
Crystallography
Niter is orthorhombic, a = 5.43, b = 9.19, c = 6.46, Z = 4; space group $ \small{P \frac{2_1}{c} \frac{2_1}{m} \frac{2_1}{n}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Niter is most common as crusts and coatings or fine dusty aggregates. When visible, niter crystals are typically acicular.
Structure and Composition
Niter is isostructural with aragonite. It forms minor solid solutions with nitratite.
Occurrence and Associations
Niter is found in arid-region soils and unconsolidated sediments in caves.
Related Minerals
Eight or nine nitrate minerals are known, but all except niter and nitratite are extremely rare.
8 Borate Minerals
|
Anhydrous Borate Group Hydrous Borate Group |
Mineralogists have identified many borate minerals. Most, especially the anhydrous borates, are very rare. Borate minerals have complex structures and chemistries, due in large part to the small size and trivalent nature of ionic boron. They have structural similarity to carbonates and nitrates because boron combines with oxygen to form anionic groups: (BO3)3- or (BO4)5-.
Borates have high solubilities in water and, consequently, are only found in relatively arid environments. They are deposited in evaporite deposits which are sometimes mined to produce boron.
▪Borax Na2B4O5(OH)4•8H2O
Origin of Name
From the Persian word burah, meaning “white.”


Hand Specimen Identification
Low specific gravity, softness, prismatic habit, solubility in water, and association help identify borax. Borax can, however, be confused with other borate minerals.
Figure 14.396 shows prismatic borax crystals, and Figure 14.397 shows scalenohedral crystals. Both specimens come from the same place in California’s Mojave Desert.
Physical Properties
| hardness | 2 to 2.5 |
| specific gravity | 1.7 to 1.9 |
| cleavage/fracture | perfect {100}, good {110}/conchoidal |
| luster/transparency | vitreous, resinous/translucent |
| color | white, gray, less commonly light blue or green |
| streak | white |
Properties in Thin Section
Borax is colorless in thin section, has three distinct cleavages, displays second-order interference colors, and shows anomalous interference colors in some orientations. Biaxial (-), a = 1.447 , β = 1.469, γ = 1.472, δ = 0.025, 2V = 40°.
Crystallography
Borax is monoclinic, a = 11.84, b = 10.63, c = 12.32, β = 106.58°, Z = 4; space group $ \small{C \frac{2}{c}} $; point group $ \small{\frac{2}{m}} $.
Habit
Euhedral borax crystals are most often stubby prisms with complex combinations of terminating faces. Borax is common in massive or granular aggregates.
Structure and Composition
Although the Na:B ratio is fixed, the amounts of (OH)– and H2O in borax are variable. The structure consists of chains of Na(H2O)6 octahedra connected to isolated groups of boron tetrahedra and double boron triangles. Weak van der Waals and hydrogen bonds link the octahedral chains to the boron groups, resulting in perfect prismatic cleavage.
Occurrence and Associations
Borax, associated with evaporite deposits in volcanic terranes, is the most common of the hydrous borate minerals. It is found in thick beds, similar to other salts, and as crusts and surface coatings. Common associated minerals are halite, NaCl; colemanite, CaB3O4(OH)3•H2O; ulexite, NaCaB5O6(OH)6•5H2O; and gypsum, CaSO4•2H2O.
Related Minerals
Borax dehydrates easily to tincalconite, Na2B4O5(OH)4•3H2O.
▪Kernite Na2B4O6(OH)2•3H O
Origin of Name
Named after its only major occurrence, in Kern County, California.


Hand Specimen Identification
Kernite commonly resembles borax and some other borate minerals (Figure 14.398). Unlike other borates, however, it forms long sometimes splintery fragments when cleaved (Figure 14.399).
Physical Properties
| hardness | 3 |
| specific gravity | 1.90 |
| cleavage/fracture | prismatic, perfect (100) and (001), poor (010)/uneven |
| luster/transparency | vitreous, pearly/transparent |
| color | colorless, white |
| streak | white |
Properties in Thin Section
Kernite is colorless in thin section and has negative relief and two perfect cleavages. Interference colors range up to second-order red or orange. Borax has a moderate 2V and lower birefringence; ulexite and colemanite have only one cleavage and are biaxial (+). Biaxial (-), a = 1.454 , β = 1.472, γ = 1.488, δ = 0.034, 2V = 80°.
Crystallography
Kernite is monoclinic, a = 15.68, b = 9.09, c = 7.02, β = 108.87°, Z = 4; space group $ \small{P \frac{2}{a}} $; point group $ \small{\frac{2}{m}}$.
Habit
Kernite typically is in massive or coarse aggregates that typically cleave into long fragments.
Structure and Composition
Kernite‘s structure is complex, consisting of mixed chains of (BO4)5- tetrahedra and (BO)3- triangles. The chains are linked by bonds to Na+ ions.
Occurrence and Associations
The only major occurrences of kernite are in Kern County, California, where it occurs with borax and ulexite.
Related Minerals
Kernite is similar to other borates in composition but, being identified by its distinctive cleavage, is rarely misidentified.
▪Ulexite NaCaB5O6(OH)6•5H2O
Origin of Name
Named for German chemist G. L. Ulex (1811–1883), who discovered it.



Hand Specimen Identification
Ulexite is similar to, and may be confused with, other borates. This mineral commonly forms soft rounded masses with a loose, “cotton ball” appearance (Figure 14.400). Less commonly it develops as prismatic, acicular or fibrous aggregates (Figure 14.401). The parallel fibrous nature of some specimens means that ulexite may transmit images like fiber optics (Figure 14.402).
Physical Properties
| hardness | 1 to 2.5 |
| specific gravity | 1.96 |
| cleavage/fracture | perfect but rarely seen {010}/uneven |
| luster/transparency | silky/transparent to translucent |
| color | white |
| streak | white |
Properties in Thin Section
Ulexite is biaxial (+), a = 1.491 , β = 1.505, γ = 1.520, δ = 0.029, 2V = 73°.
Crystallography
Ulexite is triclinic, a = 8.73, b = 12.75, c = 6.70, α = 90.27°, β = 109.13°, γ = 105.12°, Z = 2; space group $ \small{P\overline{1}} $; point group $ \small{\overline{1}}$.
Habit
Acicular and fibrous crystal aggregates are typical for ulexite. Crystals may form rounded masses with a delicate fuzzy “cotton ball” appearance. A variety called television rock contains massive, closely packed fibers, resulting in fiberoptic-like properties (Figure 14.402).
Structure and Composition
Ulexite‘s structure is complex, consisting of large B(O,OH)3 and B(O,OH)4 anionic groups, Ca2+ in 8- to 10-fold coordination, and Na+ in 6-fold coordination.
Occurrence and Associations
Similar to other borate minerals, ulexite forms in arid regions from evaporating water. It is commonly associated with borax, kernite, and colemanite.
▪Colemanite CaB3O4(OH)3•H2O
Origin of Name
Named after W. T. Coleman (1824–1893), the Californian who founded the California borax industry.

Hand Specimen Identification
Colemanite is similar in chemistry and properties to other borates and distinguishing it from the other borates can be difficult. Excellent cleavage in one direction, color, transparency, and association help identify it. Figure 14.403 shows an example of colemanite from California’s Mojave Desert.
Physical Properties
| hardness | 4 to 4.5 |
| specific gravity | 2.42 |
| cleavage/fracture | one perfect (010)/subconchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless, white, gray |
| streak | white |
Properties in Thin Section
Colemanite is colorless in thin section, appearing similar to other borates but having a higher index of refraction. Biaxial (+), a = 1.586 , β = 1.592, γ = 1.614, δ = 0.028,2V = 56°.
Crystallography
Colemanite is monoclinic, a = 8.74, b = 11.26, c = 6.10, β = 110.12°,Z = 4; space group $ \small{P \frac{2_1}{a}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Colemanite crystals vary, usually being short and prismatic but sometimes massive or granular.
Structure and Composition
Colemanite‘s structure consists of uneven sheets containing rings of (BO4)5- tetrahedra and (BO3)3-triangles.
Occurrence and Associations
Usually associated with ulexite, kernite, and borax, colemanite deposits form thick layers in ancient lake beds.
9 Sulfate Minerals
|
Anhydrous Sulfates Hydrous Sulfates |
Mineralogists divide sulfate minerals into two groups: the anhydrous sulfates and the hydrous sulfates. Chemistries and structures of the anhydrous sulfates are related to the carbonates with SO4 replacing CO3. More than 100 sulfate minerals are known, and most are rare. Gypsum and anhydrite are the only rock-forming sulfates.
Sulfates, like borates and nitrates, are common minerals in evaporite deposits. They are also, however, found as secondary minerals in many different kinds of rocks.
For more general information about sulfates, see Section 7.4.4 in Chapter 7.
▪Anhydrite CaSO4
Origin of Name
From the Greek word anhydros, because it lacks water (compared with gypsum).



Hand Specimen Identification
Anhydrite is an orthorhombic mineral with three well-developed cleavages at 90° to each other, but the most common occurrences are massive and fine-grained like the sample seen in Figure 14.404. Anhydrite may look like calcite or gypsum, but is easily distinguished from calcite by its higher specific gravity and from gypsum by its hardness.
When crystals are visible, identification is simplified. Figure 14.405 shows anhydrite crystals displaying orthorhombic symmetry and cleavage. Many coarsely crystalline anhydrite specimens, like the one seen in Figure 14.406, however, are not as clearly orthorhombic.
Physical Properties
| hardness | 3 to 3.5 |
| specific gravity | 2.98 |
| cleavage/fracture | perfect cubic (010), good (100) and (001)/uneven, splintery |
| luster/transparency | pearly, vitreous/translucent |
| color | colorless |
| streak | white |
Properties in Thin Section
Anhydrite is colorless in thin section, displays up to third-order green interference colors, has pseudocubic cleavage, and displays parallel extinction. Gypsum has lower relief and birefringence; barite and celestite have higher indices of refraction and low birefringence. Biaxial (+), a = 1.570 , β = 1.575, γ = 1.614, δ = 0.044, 2V = 44°.
Crystallography
Anhydrite is orthorhombic, a = 6.22, b = 6.97, c = 6.96, Z = 4; space group $ \small{C \frac{2}{c} \frac{2}{m} \frac{2_1}{m}} $; point group $ \small{\frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Anhydrite is usually massive, granular, or fibrous; individual crystals, typically tabular or prismatic, are not typical.
Structure and Composition
Anhydrite‘s structure is similar to zircon’s; (SO4)2- tetrahedra share edges and are linked by (CaO8) polyhedra. Anhydrite is generally close to CaSO4 in composition but may be partially hydrated (tending toward gypsum).
Occurrence and Associations
Anhydrite is typically an evaporite mineral associated with gypsum, sulfur, halite, calcite, or dolomite. Thick anhydrite beds are well known. It is also found in amygdules or cracks in basalt, as a gangue mineral in hydrothermal ore deposits, as a component of soils, or as a hot spring deposit.
Related Minerals
Anhydrite is chemically related to other anhydrous sulfates, including barite (BaSO4); celestite (SrSO4); and anglesite (PbSO4), but has a different structure. A polymorph of anhydrite, γ-CaSO4, forms when gypsum (CaSO4•2H2O) is dehydrated.
▪Barite BaSO4
Origin of Name
From the Greek word barys, meaning “heavy.”



Hand Specimen Identification
High specific gravity, white or light color, distinctive crystal habit, and two principle cleavages at 90° all help identify barite.
Figure 14.407 shows nondescript barite from Michigan’s Upper Peninsula. Most barite has a similar appearance. However, sometimes this mineral forms spectacular reticulated masses such as those seen in Figures 14.408 and 14.409. See also Figures 3.74 and 7.48. When blue, as in Figure 14.408, barite appears similar to celestite, but barite has greater density that is easily discerned by the heft test.
Figure 10.1, from a previous chapter, is a photo of spectacular blue orthorhombic barite crystals up to 50 mm tall. It is from the Huanuco Department, Peru. Figure 10.59 shows similarly shaped, but clear, crystals.
Physical Properties
| hardness | 3 to 3.5 |
| specific gravity | 4.5 |
| cleavage/fracture | perfect (001), good (010) and {210}/uneven |
| luster/transparency | vitreous, pearly/transparent to translucent |
| color | white, gray, or colorless |
| streak | white |
Properties in Thin Section
Barite is colorless in thin section and displays up to second-order yellow interference colors. Biaxial (+), a = 1.636 , β = 1.637,g = 1.648, δ = 0.012, 2V = 37°.
Crystallography
Barite is orthorhombic, a = 8.87, b = 5.45, c = 7.14, Z = 4; space group $ \small{P \frac{2_1}{n} \frac{2_1}{m} \frac{2_1}{a}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.

Habit
Barite’s crystal habit is complex and variable. Tabular crystals may combine to form cockscomb aggregates called barite roses (Figure 14.410) or crested barite (as seen in Figures 14.408 and 14.409, above). Individual rosettes are common. Barite roses appear somewhat like gypsum roses such as those in Figure 14.346. Barite is also common as massive concretions, veins, or beds.
Structure and Composition
Barite contains (SO4)2- tetrahedra linked by (BaO12) polyhedra. Each (BaO12) group is bonded to seven individual (SO4)2- tetrahedra. The structure differs from anhydrite’s.
Occurrence and Associations
Barite is a common gangue mineral in hydrothermal veins, associated with fluorite, galena, quartz, calcite, or dolomite. It is also found in veins in limestone, and as residual masses in clays.
Related Minerals
Barite is chemically and structurally similar to celestite, SrSO4, and anglesite, PbSO4, although solid solutions between the three are limited in nature.
▪Celestite SrSO4
Origin of Name
From the Latin word caelestis, meaning “celestial,” in reference to the sky-blue color of some celestite.


Hand Specimen Identification
Celestite is commonly light blue, and the blue color can be diagnostic (Figure 14.411), although it is quite similar to the color of blue calcite. Figure 7.50 shows another example of blue celestite. Celestite is sometimes associated with native sulfur (Figure 14.432). Orthorhombic crystal shape and 90° cleavage angle aid identification.
When clear, like the celestite in Figure 14.412, this mineral may resemble strontianite, another orthorhombic mineral with similar density and hardness. Some celestite resembles barite, but barite is much heavier.
Physical Properties
| hardness | 3 to 3.5 |
| specific gravity | 3.97 |
| cleavage/fracture | perfect basal (001), good prismatic {210}, poor (011)/uneven |
| luster/transparency | vitreous, pearly/transparent to translucent |
| color | colorless, blue, rarely light red |
| streak | white |
Properties in Thin Section
Celestite is similar to barite in thin section but may have a light blue color. Biaxial (+), α = 1.622 , β = 1.624, γ = 1.631, δ = 0.009, 2V = 51°.
Crystallography
Celestite is orthorhombic, a = 8.38, b = 5.37, c = 6.85, Z = 4; space group $ \small{P \frac{2_1}{n} \frac{2_1}{m} \frac{2_1}{a}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Tabular crystals, similar to those of barite, are typical for celestite. Acicular, fibrous, reniform, or granular crystals are also common.
Structure and Composition
Although in principal a complete solid solution exists between celestite (SrSO4) and barite (BaSO4), most celestite is close to end-member SrSO4. Small amounts of Pb may substitute for Sr.
Occurrence and Associations
Celestite is a rare mineral found in sedimentary rocks and in veins. Associated minerals often include dolomite, gypsum, halite, calcite, fluorite, or barite.
Related Minerals
Celestite is isostructural with barite, BaSO4, and anglesite, PbSO4. It may alter to strontianite, SrCO3, the only other Sr mineral of significance.
▪Anglesite PbSO4
Origin of Name
Named after the Welsh island of Anglesey, where it was discovered.

Hand Specimen Identification
Colorless to lightly colored translucent crystals, an adamantine luster, high density (easily discerned by the heft test), and association with galena distinguish anglesite.
Physical Properties
| hardness | 2.5 to 3 |
| specific gravity | 6.38 |
| cleavage/fracture | perfect (001), good {210}/conchoidal |
| luster/transparency | adamantine/translucent |
| color | white |
| streak | white |
Properties in Thin Section
Anglesite is biaxial (+), α = 1.877 , β = 1.883, γ = 1.894, δ = 0.017, 2V = 75°.
Crystallography
Anglesite is orthorhombic, a = 8.47, b = 5.39, c = 6.94, Z = 4; space group $ \small{P \frac{2_1}{n} \frac{2_1}{m} \frac{2_1}{a}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Anglesite is normally in blocky masses or in granular aggregates; individual crystals may be tabular, prismatic, bipyramidal, or nearly equant.
Structure and Composition
Anglesite, being isostructural with barite and celestite, may contain significant amounts of Ba or Sr.
Occurrence and Associations
Anglesite, a common alteration product of galena, PbS, is found in oxidized portions of Pb deposits. Associated minerals include cerussite, SrSO4; wulfenite, (PbMoO4); smithsonite, ZnCO3; hemimorphite, Zn4(Si2O7)(OH)2•H2O; and pyromorphite, Pb5(PO4)3Cl.
Related Minerals
There are few common Pb-minerals; galena is really the only one. Anglesite may be the second most common.
▪Gypsum CaSO4•2H2O
Origin of Name
From the Arabic word jibs, meaning “plaster.”


Hand Specimen Identification
Softness (H = 2) and three cleavages, tabular or platy crystals (like the crystals seen in Figure 14.414), and gray or white color distinguish gypsum. This mineral commonly twins; penetration and swallowtail twins (Figure 14.415) are particularly common and diagnostic. Gypsum is sometimes confused with anhydrite, a similar light-colored mineral that forms in the same environments.
The two photos above show classic gypsum; the sample in Figure 14.415 is twinned. Figure 3.17 shows a spectacular example of prismatic stellate gypsum crystals. They are quite small, but at the other end of the size scale, Figure 7.46 shows some of the largest crystals known. They are gypsum crystals that formed from hydrothermal waters in a cave in Mexico. The photo includes a person for scale.
Physical Properties
| hardness | 2 |
| specific gravity | 2.32 |
| cleavage/fracture | perfect basal (010), good (100) and {011}/conchoidal |
| luster/transparency | vitreous, pearly/transparent to translucent |
| color | colorless, white, variable |
| streak | white |
Properties in Thin Section
Gypsum is colorless in thin section and displays up to first-order yellow interference colors. It may be confused with anhydrite or barite, but anhydrite has higher relief and birefringence, and barite has higher relief and parallel extinction. Biaxial (+), α = 1.520 , β = 1.523, γ = 1.529, δ = 0.009, 2V = 58°.
Crystallography
Gypsum is monoclinic, a = 5.68, b = 15.518, c = 6.29, β = 113.83°, Z = 4; space group $ \small{A\frac{2}{n}} $; point group $ \small{\frac{2}{m}} $.

Habit
Gypsum is often massive but may form granular, acicular, or prismatic crystals. Typical gypsum crystals are tabular (like the ones seen in Figure 14.414, above), thick to thin, often forming as elongated sheaves or masses. When gypsum precipitates in wet sand, desert roses such as the ones seen in Figure 14.416 may develop. Twinning and crystal intergrowths are common.
Structure and Composition
In gypsum, layers of H2O alternate with layers containing Ca2+ and (SO4)2-. Ca2+ is bonded to six O and two H2O. The Ca2+ polyhedra link isolated (SO4)2- tetrahedra. Gypsum rarely contains significant impurities.


Occurrence and Associations
Gypsum, the most common sulfate mineral, is a rock-forming mineral of many evaporite deposits where it may be associated with other bedded salts. It is commonly found in sedimentary deposits of playa lakes (Figure 14.417). Gypsum is also found interlayered with limestones or shales and may be found in fractures or cracks in a variety of sedimentary rocks (Figure 14.418). Gypsum is a gangue mineral or alteration product in some ore deposits and is occasionally found around fumaroles.

Varieties
Well-known varieties of gypsum include selenite (clear, often needle-like, crystals), alabaster (compact white masses), and satin spar (fibrous deposits in veins) (Figure 14.419).
Related Minerals
Other hydrous sulfates include chalcanthite, CuSO4•5H2O; epsomite, MgSO4•7H2O; antlerite, Cu3SO4(OH)4; and alunite, KAl3(SO4)2(OH)6. Gypsum commonly forms from, or alters to, anhydrite, CaSO4.
▪Epsomite MgSO4•7H2O
Origin of Name
Derived from Epsom, England, where epsom salts were first precipitated from mineral waters.


Hand Specimen Identification
Typical crusty habit, low specific gravity, and salty taste are characteristic of epsomite. Figure 14.420 shows a specimen containing very fine needles of epsomite. Figure 14.421 also contain needles, but they are coarse.
Physical Properties
| hardness | 2 to 2.5 |
| specific gravity | 1.68 |
| cleavage/fracture | perfect (010), good {011}/conchoidal |
| luster/transparency | vitreous/transparent to translucent |
| color | colorless |
| streak | white |
Properties in Thin Section
Epsomite is biaxial (+), α = 1.433 , β = 1.455, γ = 1.461, δ = 0.028, 2V = 52°.
Crystallography
Epsomite is orthorhombic, a = 11.96, b = 12.05, c = 6.88, Z = 4; space group $ \small{P{2_1} {2_1} {2_1}} $; point group $ \small{ 222} $.
Habit
Epsomite may be botryoidal, fibrous, or colloform, and typically forms as crusts. Large crystals are rare.
Structure and Composition
Epsomite contains two types of H2O molecules; some are coordinated with Mg and some are not.
Occurrence and Associations
Epsomite is uncommon but occurs in caves or mine adits as encrustations, as precipitates on carbonate or mafic igneous rocks, as an evaporite mineral, or as gangue in ore deposits. It is usually associated with other sulfates.
10 Tungstates, Molybdates, and Chromates
|
wolframite series others |
Tungstates, molybdates, and chromates are chemically and structurally related to the anhydrous sulfates.
Generally rare, these minerals may be locally concentrated in ore deposits. Wolframite is the most common tungsten ore mineral. Scheelite, too, is an important tungsten ore mineral. Wulfenite is a minor molybdenum ore mineral. Crocoite is a minor secondary mineral sometimes found with other lead ore minerals.
▪Wolframite (Fe,Mn)WO4
Origin of Name
The name’s origin is unclear; perhaps it comes from the German words for “wolf” and rahm, meaning “soot,” in reference to its color. Wolframite is not a single mineral species, but is a solid solution series between end members ferberite and huebnerite.

Hand Specimen Identification
Very high specific gravity, dark color, and one good cleavage help identify wolframite. It is sometimes confused with hornblende but is much heavier. It is occasionally confused with scheelite or other heavy dark-colored minerals. Figure 14.422 shows a typical example of black wolframite. It is perched on top of clear quartz and orangish calcite.
Physical Properties
| hardness | 4 to 4.5 |
| specific gravity | 7.25 to 7.60 |
| cleavage/fracture | one perfect (010)/uneven |
| luster/transparency | submetallic/opaque unless very thin color black, red-brown |
| color | brownish black or iron black |
| streak | black, red-brown |
Properties in Thin Section
Wolframite is generally opaque. Biaxial (+), α = 2.17 to 2.31 , β = 2.22 to 2.40, γ = 2.30 to 2.46, δ = 0.13 to 0.15, 2V = 73° to 79°.
Crystallography
Wolframite is monoclinic, a = 4.71 to 4.85, = 5.70 to 5.77, c = 4.94 to 4.98, b = 90° to 91°, Z = 2; space group $ \small{P \frac{2}{c}} $; point group $ \small{\frac{2}{m}}$.
Habit
Wolframite crystals are short to long prisms or tabs, often showing vertical striations. Wolframite may form bladed, subparallel crystal groups.
Structure and Composition
A complete solid solution exists between ferberite (FeWO4) and huebnerite (MnWO4), the two principal wolframite end members. The basic structure consists of layers of distorted (WO4)2- tetrahedra joined by octahedral Fe or Mn.
Occurrence and Associations
Wolframite is a rare mineral. Usually found in high-temperature quartz veins associated with granitic igneous rocks, it is, however, the most important tungsten ore mineral. It is also found in sulfide-rich veins, associated with scheelite, cassiterite, pyrite, or galena. It may contain minor amounts of Ca, Mg, or rare earth elements.
Related Minerals
Similar minerals include the rare tungstates sanmartinite, (Zn,Fe)WO4, and raspite, PbWO4.
▪Scheelite CaWO4
Origin of Name
Named after K. W. Scheele (1742–1786), a Swedish chemist.

Hand Specimen Identification
High specific gravity, crystal habit, distinct cleavage, and fluorescence under ultraviolet light are characteristic for scheelite. It may be confused with quartz or feldspar when uncolored.
Figure 14.423 shows scheelite crystals, with sheelite‘s classic orange diagnostic color, on quartz and muscovite. Scheelite, however, may have other hues.
Physical Properties
| hardness | 4.5 to 5 |
| specific gravity | 6.11 |
| cleavage/fracture | good pyramidal {101}, poor {112}/uneven |
| luster/transparency | subadamantine/transparent to translucent |
| color | tan, orange, brown, yellow, white, or colorless |
| streak | white |
Properties in Thin Section
Scheelite is uniaxial (+), ω = 1.920, ε = 1.934, δ = 0.014.
Crystallography
Scheelite is tetragonal, a = 5.25, c = 11.40, Z = 4; space group $ \small{I \frac{4_1}{a}} $; point group $ \small{ \frac{4}{m}} $.
Habit
Scheelite is found in massive, columnar, or granular aggregates and as individual crystals, usually dipyramids.
Structure and Composition
Scheelite is usually close to end-member composition, but a limited solid solution exists with powellite, CaMoO4. It may also incorporate small amounts of Cu or Mn. In the structure, isolated (WO4)2- tetrahedra are linked by 8-coordinated Ca.
Occurrence and Associations
Scheelite is a high-temperature mineral found in metamorphic aureoles, in granites and pegmatites, and in some hydrothermal veins. It may be found with cassiterite, SnO2; topaz, Al2SiO4(F,OH)2; fluorite, CaF2; apatite, Ca5(PO4)3(OH,F,Cl); and with other tungstates or molybdates.
Related Minerals
Scheelite is isostructural with wulfenite, PbMoO4.
▪Wulfenite PbMoO4
Origin of Name
Named after Austrian mineralogist F. X. von Wulfen (1728–1805).


Hand Specimen Identification
Crystal habit and orange-yellow color are distinctive. Wulfenite is occasionally confused with native sulfur but has much greater density.
Figure 14.424 shows a specimen of wulfenite from southern Arizona. Figure 14.425 is a photo of a display case with a dozen samples from different places. The orange to yellow-orange color, although a bit variable, is a key to identifying this mineral.
Physical Properties
| hardness | 3 |
| specific gravity | 6.7 to 7.0 |
| cleavage/fracture | good but rarely seen pyramidal {011}, poor (001)/ subconchoidal |
| luster/transparency | adamantine, silvery/ transparent to translucent |
| color | yellow, orange, red, brown, green |
| streak | white |
Optical Properties
Wulfenite is uniaxial (-), ω = 2.404, ε = 2.283, δ = 0.121.
Crystallography
Wulfenite is tetragonal, a = 5.42, c = 12.10, Z = 4; space group $ \small{I \frac{4_1}{a}} $; point group $ \small{ \frac{4}{m}} $.
Habit
Wulfenite crystals are most often square tablets, often thin or pyramidal. They may also be prismatic.
Occurrence and Associations
Wulfenite is a rare secondary mineral found in the oxidized portions of Pb deposits. It may be associated with galena, PbS; cerussite, PbCO3; vanadinite, Pb5(VO4)3Cl; or pyromorphite, Pb5Cl(PO4)3. Wulfenite is isostructural with scheelite, CaWO4, with which it forms limited solid solutions. It also forms limited solid solutions with powellite, CaMoO4, and raspite, PbWO4.
▪Crocoite PbCrO4
Origin of Name
From the Greek word krokos, meaning “saffron,” referring to its color.


Hand Specimen Identification
Distinctive reddish-orange color and adamantine luster, its high specific gravity and habit, and association with other Pb-minerals usually make crocoite easy to identify. It is occasionally confused with wulfenite, PbMoO4, or with vanadinite, Pb5(VO4)3Cl, both of which may have strong red or orange colors.
Figure 14.426 shows crocoite on goethite from Australia. Figure 14.427 shows crocoite from the Ural Mountains, Russia. See also the photo of crocoite with pyromorphite and galena in Figure 14.433.
Physical Properties
| hardness | 2.5 to 3 |
| specific gravity | 6.0 |
| cleavage/fracture | perfect {110}, poor (001)/subconchoidal |
| luster/transparency | adamantine/translucent |
| color | reddish orange |
| streak | orange |
Optical Properties
Crocoite is biaxial (+), α = 2.31 , β = 2.37, γ = 2.66, δ = 0.35, 2V = 54°.
Crystallography
Crocoite is monoclinic, a = 7.11, b = 7.41, c = 6.81, β = 102.55°, Z = 4; space group $ \small{P \frac{2_1}{n} } $; point group $ \small{\frac{2}{m} } $.
Habit
Crocoite crystals are generally acicular or columnar. The thin prismatic crystals often have striations parallel to prism faces. More rarely, crocoite forms granular aggregates or patches.
Structure and Composition
Crocoite is isostructural with monazite, (Ce,La,Th,Y)PO4. It consists of distorted (CrO4)2- tetrahedra alternating with Pb in 9-fold coordination.
Occurrence and Associations
Crocoite is a rare secondary Pb mineral associated with veined lead deposits. It may be found with cerussite, PbCO3; pyromorphite, Pb(PO4)3Cl; or wulfenite, PbMoO4.
Related Minerals
Crocoite is isostructural with monazite, (Ce,La,Th,Y)PO4, and with several other rare earth silicates and phosphates. It is closely related to xenotime, YPO4, and pucherite, BiVO4.
11 Phosphates, Arsenates, and Vanadates
The phosphate group contains many minerals, but most are extremely rare. Apatite is the only common example. Vanadates and arsenates, which are closely related to the phosphates in chemistry and structure, are also rare.
| Phosphate Group Minerals monazite (Ce,La,Th,Y)PO4 triphylite Li(Fe,Mn)PO4 apatite Ca5(PO4)3(OH,F,Cl) pyromorphite Pb5(PO4)3Cl amblygonite LiAl(PO4)F lazulite (Mg,Fe)Al2(PO4)2(OH)2 wavellite Al3(PO4)2(OH)3•5H2O turquoise CuAl6(PO4)4(OH)8•4H2O autunite Ca(UO2)2(PO4)2•10H2O |
Vanadate Group Minerals Arsenate Group Minerals
|
▪Monazite (Ce,La,Th,Y)PO4
Origin of Name
From the Greek word monazein, meaning “to live alone,” referring to its rare occurrences in the outcrops where it was first found.


Hand Specimen Identification
Radioactivity, color, crystal habit, and associations help identify monazite. It may be confused with zircon, but is not as hard and has different forms. It can be distinguished from titanite by its crystal shape and high density.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 4.9 to 5.2 |
| cleavage/fracture | perfect (001), good (100)/subconchoidal |
| luster/transparency | variable, subresinous/translucent |
| color | red, brown, yellowish |
| streak | white |
Optical Properties
Monazite is colorless, gray, or yellow-brown in thin section. It has high positive relief and displays up to third or fourth order interference colors. Biaxial (+), α = 1.785 to 1.800 , β = 1.786 to 1.801, γ = 1.838 to 1.850, δ = 0.005, 2V = 10° to 20°.
Crystallography
Monazite is monoclinic, a = 6.79, b = 7.01, c = 6.46, β = 104.4°, Z = 4; space group $ \small{P \frac{2_1}{n} } $; point group $ \small{\frac{2}{m} } $.
Habit
Monazite crystals are usually small, tabular, or prismatic, often forming granular masses or individual grains in sand.
Structure and Composition
Monazite is isostructural with crocoite. In its structure, distorted (PO4)3- polyhedra are bonded to rare earth elements in 9-fold coordination. All the rare earths may be present, but Ce, La, and Th are usually the dominant large cations. Small amounts of Si may substitute for P in the tetrahedral sites.
Occurrence and Associations
Monazite is a rare secondary mineral is silicic igneous rocks. It is also found in unconsolidated beach or stream sediments, where it is associated with other heavy minerals such as magnetite and ilmenite.
Varieties
Rare earth content varies, so the names monazite-(Ce), monazite-(La), and so on are sometimes used to designate the dominant rare earth.
Related Minerals
Monazite is isostructural with crocoite, PbCrO4, and forms solid solutions with huttonite, ThSiO4. It is chemically related to xenotime, YPO4, with which it forms a minor solid solution.
▪ Triphylite Li(Fe,Mn)PO4
Origin of Name
From the Greek words for “three” and “family,” in reference to its three cations.

Hand Specimen Identification
Brown-green to gray or bluish color, association with other pegmatite minerals, 90° cleavage angle, and resinous luster help identify triphylite.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 3.5 to 5.5 |
| cleavage/fracture | perfect (001), good (010)/subconchoidal |
| luster/transparency | vitreous, resinous/ transparent to translucent |
| color | variable brown-green to gray or bluish |
| streak | white, gray |
Optical Properties
Triphylite is biaxial (-), α = 1.68 , β = 1.68, γ = 1.69, δ = 0.01, 2V = 0° to 56°.
Crystallography
Triphylite is orthorhombic, a = 6.01, b = 4.68, c = 10.36, Z = 4; space group $ \small{P \frac{2_1}{m} \frac{2_1}{c} \frac{2_1}{n}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
Euhedral crystals are rare; triphylite is typically fine grained and massive.
Structure and Composition
In triphylite, the cations occupy octahedra forming zigzag chains between (PO4)3- tetrahedra. A complete solid solution exists between the Fe and Mn end members. Compositions near the Mn end member are given the name lithiophilite.
Occurrence and Associations
Triphylite, typically a pegmatite mineral, is found with other phosphates, quartz, feldspar, spodumene, and beryl.
▪Apatite Ca5(PO4)3(OH,F,Cl)
Origin of Name
From the Greek word apate, meaning “deceit,” because it is often difficult to distinguish it from other minerals.


Hand Specimen Identification
Apatite may be any of a number of different colors. The olive-green color seen in Figure 14.431 is most common. Other shades of green and blue are quite common, too, such as the blue color of the crystals in Figure 14.432. Other hues are relatively rare.
When euhedral, apatite crystals are easy to discern hexagonal prisms, often with terminating pyramidal faces. Cleavage traces run perpendicular to prism faces and long dimension. (Cleavage is quite obvious in Figure 14.432) Also aiding identification: apatite has moderate density, is softer than quartz and feldspar, but harder the calcite and fluorite.
Physical Properties
| hardness | 5 |
| specific gravity | 3.2 |
| cleavage/fracture | good (001), poor (100)/conchoidal |
| luster/transparency | subresinous/transparent to translucent |
| color | green, yellow, variable |
| streak | white |
Optical Properties
Apatite appears similar to quartz in thin section. It is colorless, does not develop good cleavage, and has very low birefringence. Quartz, however, has lower relief and is uniaxial (+). Apatite is uniaxial (-), ω = 1.633, ε = 1.630, δ = 0.003.
Crystallography
Apatite is hexagonal, a = 9.38, c = 6.86, Z = 2; space group $ \small{P \frac{6_3}{m}} $; point group $ \small{ \frac{6}{m}} $.
Habit
Apatite typically forms prismatic crystals, but may be colloform, massive, or granular.
Structure and Composition
Complete solid solution exists between hydroxyapatite (OH end member), fluorapatite (F end member), and chlorapatite (Cl end member). In addition, transition metals or Sr2+ may replace Ca2+; and (CO3)2-, OH–, or (SO4)2- may replace some (PO4)3-. In the apatite structure, Ca-PO4 chains run parallel to the c-axis. Ca2+ is located around channels occupied by (F,Cl,OH).
Occurrence and Associations
Apatite is a common accessory mineral but only rarely a major rock former. It is common in all igneous rocks, including pegmatites and hydrothermal veins, in metamorphic rocks, and in marine sediments.
Varieties
Collophane is a massive cryptocrystalline form of apatite that comprises some phosphate rocks and bones.
Related Minerals
A large number of phosphates, sulfates, arsenates, vanadates, and silicates are isostructural with apatite, but none are common.
▪Pyromorphite Pb5(PO4)3Cl
Origin of Name
From the Greek words meaning “fire” and “form,” because it typically develops large faces when crystallizing from a magma.


Hand Specimen Identification
Occurrence with other Pb-minerals, bright green, green-yellow, or brown color, habit, high density, and sometimes resinous luster identify pyromorphite. It is sometimes confused with apatite, mostly because of its green color, but is softer than apatite.
Figure 14.433 shows green pyromorphite on gray galena (Pb-sulfide). Minor orange crocoite (Pb-chromate) can also be seen. The specimen in Figure 14.434 contains green pyromorphite and white/clear cerussite (Pb-carbonate). The brown material around the edges may be crocoite.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 7.0 |
| cleavage/fracture | poor {100}, poor {101} |
| luster/transparency | resinous/transparent to translucent |
| color | bright green, yellow, variable |
| streak | white, yellow |
Optical Properties
Pyromorphite is uniaxial (-), ω = 2.058, ε = 2.048, δ = 0.010.
Crystallography
Pyromorphite is hexagonal, a = 9.97, c = 7.32, Z = 2; space group $ \small{P \frac{6_3}{m}} $; point group $ \small{ \frac{6}{m}} $.
Habit
Pyromorphite is typically prismatic, having a barrel shape; it is less commonly granular, fibrous, cavernous (hollow prisms), globular, or reniform.
Structure and Composition
Pyromorphite is isostructural with apatite (see apatite structure). Some Ca may substitute for Pb. P may be replaced by As.
Occurrence and Associations
Pyromorphite is a secondary mineral, found with other oxidized Pb or Zn minerals, in oxidized zones associated with Pb deposits. It is isostructural with apatite and with mimetite, Pb(AsO4)3Cl, with which it forms a complete solid solution.
▪Amblygonite LiAl(PO4)F
Origin of Name
From the Greek word amblygonios, referring to its cleavage angle.

Hand Specimen Identification
Amblygonite is most often a nondescript white to creamy translucent mineral that is difficult to distinguish from other light-colored minerals with moderate density and hardness. Its single perfect cleavage and conchoidal fracture help identification and distinguish it from feldspars, feldspathoids, and zeolites. Its occurrence in pegmatites, especially if it contrasts with any feldspar present, also aids identification. Figure 14.435 shows typical nondescript amblygonite. Figure 10.64 (from an earlier chapter) shows an unusual yellow specimen.
Physical Properties
| hardness | 6 |
| specific gravity | 3.0 |
| cleavage/fracture | perfect (100), good (110), poor (011)/ subconchoidal |
| luster/transparency | vitreous, greasy/transparent to translucent |
| color | white, beige, green, sometimes other colors |
| streak | white |
Optical Properties
Amblygonite is biaxial (-), α = 1.59 , β = 1.60, γ = 1.62, δ = 0.03, 2V = 52° to 90°.
Crystallography
Amblygonite is triclinic, a = 6.644, b = 7.744, c = 6.91, α = 90.35°, β = 117.33°, γ = 91.01°, Z = 2; space group $ \small{P\overline{1}} $; point group $ \small{\overline{1}}$.
Habit
Rare crystals may be equant or columnar. Amblygonite is more commonly found as rough masses or in irregular aggregates.
Structure and Composition
Amblygonite is composed of alternating (PO4) tetrahedra and AlO5F octahedra linked by Li+ ions. F may be replaced by OH. Minor Na may be present.
Occurrence and Associations
Amblygonite is a rare mineral found in pegmatites rich in Li and P. Typical associated minerals include lepidolite, spodumene, apatite, and tourmaline.
Related Minerals
Several other phosphate minerals, some which can be gems, are similar to amblygonite and are found in pegmatites: herderite, CaBe(PO4)(F,OH); beryllonite, NaBePO4; and brazilianite, NaAl3(PO4)2(OH)4. Complete solid solution exists between amblygonite and a hydroxy end member, montebrasite, LiAl(PO4)(OH).
▪Lazulite (Mg,Fe)Al2(PO4)2(OH)2
Origin of Name
From an Arabic word meaning “heaven,” referring to its sky-blue color.


Hand Specimen Identification
Lazulite’s azure-blue color is distinctive. Few minerals ever have a strong blue color like lazulite‘s. When crystals are visible, their pyramidal form distinguishes lazulite from the other blue minerals. When massive, identification is problematic.
Figure 14.436 shows typical lazulite crystals, up to several centimeters long, from Austria. The light blue color is typical. Some lazulite has a darker color; the photo of the specimen from the Yukon in Figure 14.437 shows an example.
Physical Properties
| hardness | 5 to 5.5 |
| specific gravity | 3.0 |
| cleavage/fracture | poor (011)/uneven |
| luster/transparency | vitreous/translucent |
| color | azure-blue |
| streak | white |
Optical Properties
Lazulite is biaxial (-), α = 1.612 , β = 1.634, γ = 1.643, δ = 0.031, 2V = 70°.
Crystallography
Lazulite is monoclinic, a = 7.16, b = 7.26, c = 7.24, β = 120.67°, Z = 2; space group $ \small{P \frac{2_1}{c}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Lazulite is typically massive, granular, or compact; rare crystals are prismatic or pyramidal with steep faces.
Structure and Composition
Octahedral Mg and Fe are linked to octahedral Al by sharing of O2- and OH–. The (Mg,Fe,Al)6 octahedra bond to (PO4)3- tetrahedra. Complete solid solution exists between the Mg and Fe end members. Scorzalite is the name of Fe-rich members of the series.
Occurrence and Associations
Lazulite and related minerals are rare, found only in some pegmatites and high-grade metamorphic rocks. They may be associated with rutile, kyanite, corundum, and sillimanite.
▪Wavellite Al3(PO4)2(OH)3•5H2O
Origin of Name
Named after W. Wavel (d. 1829), who discovered it.


Hand Specimen Identification
Wavellite is a secondary mineral that most commonly forms planar radiating clusters of crystals along fracture surfaces, creating “starburst” structures like those seen in Figure 14.438. Wavellite also forms globular radiating aggregates of acicular crystals that develop in voids. This produces spherical structures with a “cotton-ball” appearance, like the wavellite balls seen in Figure 14.439. The planar or cotton-ball radiating textures, and a light green to yellow color, are diagnostic for this mineral. Other occurrences are known, but not as easily identified as wavellite.
Physical Properties
| hardness | 3.5 to 4 |
| specific gravity | 2.36 |
| cleavage/fracture | perfect prismatic {101}, perfect (010)/subconchoidal |
| luster/transparency | vitreous/translucent |
| color | white, greenish yellow, gray, brown |
| streak | white |
Optical Properties
Wavellite is biaxial (+), α = 1.525 , β = 1.534, γ = 1.552, δ = 0.027, 2V = 72°.
Crystallography
Wavellite is orthorhombic, a = 9.62, b = 17.36, c = 6.99, Z = 4; space group $ \small{P \frac{2_1}{c} \frac{2_1}{m} \frac{2_1}{n}} $; point group $ \small{ \frac{2}{m} \frac{2}{m} \frac{2}{m}} $.
Habit
2-dimensional or 3-dimensional radiating acicular crystal aggregates typify wavellite. Less commonly wavellite forms dense opal-like or stalactitic masses. Large visible individual crystals are very rare.
mo
Structure and Composition
Wavellite’s structure is incompletely known. It may contain small amounts of Fe and Mg.
Occurrence and Associations
Wavellite is a secondary mineral found in rock cavities or on joint surfaces in low-grade aluminous metamorphic rocks. It is also found in phosphorite deposits. Common associated minerals include other phosphate minerals and limonite.
▪Turquoise CuAl6(PO4)4(OH)8•4H2O
Origin of Name
From the French word turquoise, meaning “Turkish,” in reference to the source of the original stones imported into Europe.


Hand Specimen Identification
Turquoise forms in veins or as void fillings and is generally cryptocrystalline (exceptionally fine- grained), sometimes appearing amorphous. It typically has a distinctive turquoise-blue color seen in these two photos, but different shades of blue and green are well known. Its color and cryptocrystalline character generally serve for identification.
Turquoise is not often confused with other minerals. Chrysocolla, a blue-green hydrated Cu-silicate may have similar colors but is much softer. (Chrysocolla has hardness of 2-3 compared with turquoise‘s 6.) Malachite (green) and azurite (blue), both hydrated copper carbonates, have colors distinct from turquoise and hardnesses of 3.5 to 4.
Physical Properties
| hardness | 6 |
| specific gravity | 2.7 |
| cleavage/fracture | perfect but rarely seen (001), good (010)/subconchoidal, brittle |
| luster/transparency | resinous, waxy/translucent |
| color | turquoise and related blue-green shades, other hues are exceptionally rare |
| streak | white, green |
Optical Properties
Turquoise is biaxial (+), α = 1.61, β = 1.62, γ = 1.65, δ = 0.04, 2V = 40°.
Crystallography
Turquoise is triclinic, a = 7.48, b = 9.95, c = 7.69, α = 111.65°, β = 115.38°, γ = 69.43°, Z = 1; space group P1; space group $ \small{P1} $; point group $ \small{1}$.
Habit
Rare small crystals of turquoise are known, but reniform, massive, or granular varieties are more typical.
Structure and Composition
The structure of turquoise consists of a framework of (PO4)3- tetrahedra and Al octahedra. Holes in the structure contain Cu, which bonds to the polyhedra, OH– and to H2O. Fe3+ may substitute for Al3+.
Occurrence and Associations
Turquoise occurs as a secondary mineral associated with Al-rich volcanic rocks. It forms in small seams, veins, stringers, and crusts. Associated minerals typically include kaolinite, Al2Si2O5(OH)4; limonite, Fe2O3•nH2O; or chalcedony, SiO2.
Varieties
Zn-rich blue-green varieties of turquoise are called faustite.
Related Minerals
Complete solid solution exists between turquoise and chalcosiderite, CuFe6(PO4)4(OH)8•4H2O.
▪Autunite Ca(UO2)2(PO4)2•10H2O
Origin of Name
Named for Autun, France, where it is found.


Hand Specimen Identification
A strong yellow or yellow-green color, radioactivity, fluorescence under ultraviolet light, and typically tetragonal platy crystals identify autunite.
Figure 14.442 shows autunite from France. Figure 14.443 show a reticulated cluster of autunite crystals from Portugal.
Physical Properties
| hardness | 2 to 2.5 |
| specific gravity | 3.15 |
| cleavage/fracture | perfect basal (001), good prismatic (100), (010) and {110 }/uneven |
| luster/transparency | pearly, adamantine/transparent to translucent |
| color | typically lemon yellow to greenish yellow to pale green |
| streak | yellow |
Optical Properties
Autunite is uniaxial (-), ω = 1.577, ε = 1.553, δ = 0.024.
Crystallography
Autunite is tetragonal, a = 7.00, c = 20.67, Z = 4; space group $ \small{I \frac{4}{m} \frac{2}{m} \frac{2}{m}} $; point group $ \small{ \frac{4}{m} \frac{2}{m} \frac{2}{m}} $
Habit
Thin tabular or flaky crystals, often square, are typical for autinite. It commonly forms reticulated masses of crusts.
Structure and Composition
In autunite, (PO4)3- tetrahedra link to U octahedra to form uneven layers. The layers are connected by weakly bonded H2O molecules. Other alkaline earths may substitute for Ca in small amounts; the amount of water in the structure is somewhat variable.
Occurrence and Associations
Autunite is a secondary uranium mineral, often forming after uraninite, UO2. Torbernite, Cu(UO2)2(PO4)2•nH2O, and uraninite are common associated minerals.
Related Minerals
Torbernite is isostructural with autunite and has similar properties.
▪Vanadinite Pb5(VO4)3Cl
Origin of Name
Named in reference to its vanadium content.


Hand Specimen Identification
Vanadinite generally has a distinctive bright red to orange-red color and forms hexagonal crystals. Color is the key to identification and distinguishes this mineral from apatite, pyromorphite, or mimetite. It may be transparent, translucent, or opaque and has an adamantine to resinous luster.
Figure 14.444 shows a typical occurrence of vanadinite with calcite from Mexico. Figure 14.445 is a photo of a spectacular museum specimen that originally came from Morocco. Enlarge this photo and the hexagonal nature of the crystals is easily seen.
Physical Properties
| hardness | 3 |
| specific gravity | 6.9 |
| cleavage/fracture | none/subconchoidal |
| luster/transparency | resinous/translucent |
| color | ruby red, orange-red, brown, yellow |
| streak | white, yellow |
Optical Properties
Vanadinite is uniaxial (-), ω = 2.416, ε = 2.350, δ = 0.066.
Crystallography
Vanadinite is hexagonal, a = 10.33, c = 7.35, Z = 2; space group $ \small{P \frac{6_3}{m}} $; point group $ \small{ \frac{6}{m}} $.
Habit
Vanadinite often forms hexagonal prisms, sometimes hollow, with or without pyramidal faces. It may also form rounded or globular masses.
Structure and Composition
Vanadinite has the same structure as apatite (see apatite structure). P or As may substitute for V in small amounts. Minor amounts of Ca, Zn, and Cu may also be present.
Occurrence and Associations
Vanadinite is a rare mineral found in the oxidized portions of Pb deposits where it is often associated with galena, cerussite, or limonite.
Related Minerals
Vanadinite is isostructural with apatite, Ca5(PO4)3(OH,F,Cl), and with a number of other arsenates, vanadates, and phosphates. It forms solid solutions with mimetite, Pb5(AsO4)3Cl, and intermediate compositions are called endlichite.
▪Carnotite K2(UO2)2(VO4)2•3H2O
Origin of Name
Named after M. A. Carnot (1839–1920), a French mining engineer and Inspector General of Mines.


Hand Specimen Identification
Radioactivity and yellow color, often with a greenish stain, characterize carnotite. It is sometimes confused with other secondary uranium minerals.
Figure 14.446 shows typical fine-grained carnotite deposited in a sandstone. Individual crystals are exceptionally small. Figure 14.447 is a much magnified photo of carnotite crystals smaller than millimeter across.
Physical Properties
| hardness | 1 |
| specific gravity | 4.5 |
| cleavage/fracture | perfect but rarely seen (001)/uneven |
| luster/transparency | dull, earthy/translucent |
| color | yellow, yellow-green |
| streak | yellow |
Optical Properties
Carnotite is biaxial (-), α = 1.75 , β = 1.92, γ = 1.95, δ = 0.20, 2V = 38° to 44°.
Crystallography
Carnotite is monoclinic, a = 10.47, b = 8.41, c = 6.91, β = 103.67°, Z = 1; space group $ \small{P \frac{2_1}{a}} $; point group $ \small{ \frac{2}{m}}$.
Habit
Fine powder or crumbly aggregates characterize carnotite. It may also be disseminated.
Structure and Composition
Carnotite‘s structure contains layers of edge-sharing uranium and vanadium polyhedra. The layers are joined by weak bonds to interlayer K and H2O.
Occurrence and Associations
Carnotite is a secondary uranium mineral typically found as crusts or flakes in sandstones or conglomerates that have been altered by circulation of meteoric waters.
Related Minerals
A number of other hydrated uranium oxides are known, including tyuyamunite, Ca(UO2)2(VO4)2•nH2O; torbernite, Cu(UO2)2(PO4)2•nH2O; and autunite, Ca(UO2)2(PO4)2 •10H2O.
▪Erythrite Co(AsO)2•8H2O
Origin of Name
From the Greek word erythros, meaning “red.”


Hand Specimen Identification
Erythrite is a secondary cobalt-arsenic mineral. It is one of only a few minerals that have its kind of red-pink-purple color; the two photos here show examples. Color, association with other cobalt minerals, and often crusty or drusy appearance generally identify this mineral.
Figure 14.448 is a photo of typical crusty erythrite. The specimen comes from Australia. Figure 14.449 shows a much enlarged view of a similar specimen from Morocco that contains both needles and cottony balls of erythrite. The largest needles and balls are several millimeters in longest dimension.
Physical Properties
| hardness | 1.5 to 2.5 |
| specific gravity | 3.06 |
| cleavage/fracture | perfect basal (010)/sectile |
| luster/transparency | adamantine/transparent to translucent |
| color | crimson, pink, purple-red |
| streak | pale purple |
Optical Properties
Erythrite is biaxial (-), α = 1.626, β = 1.661, γ = 1.699, δ = 0.073, 2V = 90°.
Crystallography
Erythrite is monoclinic, a = 10.26 , β = 13.37, c = 4.74, β = 105.1°, Z = 2; space group $ \small{C \frac{2}{m}} $; point group $ \small{\frac{2}{m}} $.
Habit
Large, easily seen crystals are rare. Erythrite is typically in prismatic, acicular, reniform, or globular groups/clusters. Erythrite typically forms as drusy coatings and crusts and may be earthy or powdery.
Structure and Composition
The atomic structure in erythrite is layered, with vertex sharing by As tetrahedra and Co octahedra. Ni may substitute for Co.
Occurrence and Associations
Erythrite may form as a pink powdery coating, called cobalt bloom, on other cobalt minerals such as cobaltite, (Co,Fe)AsS, or skutterudite, (Co,Ni)As3.
Related Minerals
Annabergite, Ni3(AsO4)2•8H2O, also called nickel bloom, is isostructural with erythrite, but has an apple-green color. A complete solid solution exists between erythrite and annabergite.
Figure Credits(opening photo) Rhodochrosite, quartz, chalcopyrite (Robert M. Lavinsky, Wikimedia Commons) |


































