1.1 Blue cavansite (a calcium-vanadium silicate) on top of silvery heulandite (calcium-sodium aluminosilicate)
1 Introduction
KEY CONCEPTS
- Minerals make up just about everything on our planet and are important for maintaining our lifestyles.
- Minerals in rocks or sediment make up the material we walk on and form the foundations for buildings and other structures.
- Mineral resources have long been used by people; today they are keys to modern agriculture and industry.
- The knowledge gained by studying minerals has wide ranging applications.
- Most minerals are naturally occurring homogeneous geological substances that are stable at Earth-surface conditions.
- Minerals are inorganic compounds and are crystalline solids.
- Non-crystalline materials, synthetic minerals, biological minerals, and anthropogenic minerals are not, in general, considered to be true materials.
- We generally classify minerals based on their chemical compositions and atomic arrangements.
1.1 The Importance of Minerals

Minerals are our planet. They form the Earth and the bedrock that we live on, making up all of Earth’s rocks and sediments, and they are important components in soils. So, they literally are the foundations for our lives. Perhaps because they are ubiquitous, most people don’t even notice them or consider that all rock is made of minerals. But, engineers do because building a bridge or other structure on unstable material, or using poor ingredients for construction of all sorts, would lead to disasters. And, farmers care about minerals because healthy soils produce great crops. Petrologists who study rocks of all sorts need to know about minerals. And others who use resources in manufacturing need minerals. So, the world’s people rely on minerals. And, minerals, mineral production, and the study of minerals are absolutely essential to maintain our lifestyles.

The use and processing of minerals goes back more than 4,000 years. In fact, archaeologists and anthropologists define major periods of early human civilization based on mineral resources that were used. The late Stone Age, also called the Neolithic Age, was followed by the Chalcolithic Age from 4,500 to 3,500 BCE (Before Common Era) when people started using native copper to make tools and other artifacts. The Bronze Age that followed the Chalcolithic Age began in the Mesopotamian civilization of Sumeria and lasted from 4,200 to 1,000 BCE. During this time people combined mineralogical tin and copper and the result was bronze – a metal alloy that was stronger and more durable than copper. Figure 1.3 shows some Bronze Age artifacts. Human progress was not the same everywhere, and some early cultures progressed to the Chalcolithic and Bronze Ages before others. Even today, some Stone Age cultures still exist.
The Iron Age followed the Bronze Age, beginning around 1,500 BCE, when the Hittite society of ancient Anatolia (modern day Turkey) discovered how to smelt iron. The iron came from native iron in meteorites that also contained small amounts of nickel. Fortuitously, the nickel produced an alloy superior to pure iron. So, copper, tin, iron, and nickel were all important to the lives of early humans. They are equally important today. These metals – and many others – are key parts of a seemingly infinite number of products we use every day. The metals come from minerals.
Ask most people today why minerals are important, and they will probably mention diamonds and other gems, or perhaps precious metals such as gold or silver. Gems and gold are important, but other minerals resources are equally, or substantially more, important. Highways and buildings, fertilizers, cars, jewelry, computers and other electronic devices, kitchenware, salt, magazines, vitamins and medicines, and just about everything that supports modern living require mineral resources for production. We are addicted to minerals and related commodities.
1.1.1 Mineral Commodities
The table below, from the United States Geological Survey, lists some mineral commodities deemed essential for modern living, and the amounts that a typical person consumes in their lifetime. The list includes both metals derived from minerals (some minerals are listed in the right-hand column) and rock materials, such as stone or sand, that are made of minerals.
| Metallic Commodities |
||
| metal | lifetime needs | primary ore mineral |
| aluminum (bauxite) | 5,677 pounds | bauxite |
| cement | 65,480 pounds | calcite in limestone or related rocks |
| clay minerals | 19,245 pounds | clays in sedimentary deposits |
| copper | 1,309 pounds | copper sulfide minerals |
| gold | 1,576 ounces | native gold in hard rock or sediments |
| iron | 29,608 pounds | magnetite and hematite |
| lead | 928 pounds | galena |
| phosphate rock | 19,815 pounds | apatite |
| stone, sand, and gravel | 1.61 million pounds | mineral mixtures in sedimentary deposits |
| zinc | 671 pounds | sphalerite |
Overall, modern society uses stone, sand, gravel and other construction materials more than other mineral commodities. Construction materials are generally mixtures of different minerals and are prized for their overall properties, not the properties of the individual mineral components. We also use large amounts of what are termed industrial minerals – resources valued for their mineralogical properties. Industrial minerals include limestone, clays, bentonite, silica, barite, gypsum, and talc.
1.1.2 Ore Minerals
| Ore Minerals |
| aluminum bauxite (mix of Al-hydroxides) |
| copper bornite Cu5FeS4 chalcocite Cu2S chalcopyrite CuFeS2 malachite Cu2CO3(OH)2 |
| chrome chromite (Fe,Mg)Cr2O4 |
| lead galena PbS |
| iron hematite Fe2O3 ilmenite FeTiO3 magnetite Fe3O4 |
| molybdenum molybdenite MoS2 |
| manganese pyrolusite MnO2 |
| tungsten scheelite CaWO4 wolframite (Fe,Mn)WO4 |
| zinc sphalerite ZnS |
Ore minerals are especially significant to our daily lives. These are minerals that are mined and processed for the elements they contain. Some of the most important elements include iron, aluminum, copper, zinc, and other metals associated with steel making and modern industry. The table to the right lists common ore minerals for a number of important metals. These minerals are prized because they contain a large amount of the desired metal and because processing to remove the metal is relatively easy.

This photo (Figure 1.4) shows a rock containing the ore minerals azurite (blue) and malachite (green). The specimen comes from the Morenci Mine, the most productive of about a dozen copper mines in Arizona. Arizona produces more copper than any other U.S. state. The United States, however, is in 5th place when it comes to copper production. Copper is mined around the world, and Chile is the biggest producer by far.
People produce and use huge amounts of copper, iron, aluminum, and some other metals, but production statistics do not reflect the true importance of some mining operations. Small amounts of rare elements, for example gallium, indium, and selenium, derived from equally rare minerals, and only produced in small quantities, are keys to fast computers, smart phones, and other cutting-edge devices.
Minerals are produced, bought, and sold on a world market. And, mineral resources are not everywhere. So, today, the United States does not produce gallium, indium, selenium, and some other metals in sufficient amounts to meet our needs. We are entirely dependent on imports for about 20 important mineral commodities.
1.1.3 The Importance of Crystallography
So, we prize mineral commodities and ore minerals because they provide resources that support our lifestyles. But, the science of mineralogy is important in other ways. In particular, crystallography, the branch of mineralogy that deals with the formation and properties of crystals, plays a huge role in our lives. This is because knowledge of crystals and their properties, and the technology that comes from them, are fundamental to electronics, and to modern living.

For example, the silicon chips in electronic devices (Figure 1.5), semiconductors and microchips of many sorts, and the LCD (liquid crystal display) screen on a smart phone are all crystalline. Light emitting diodes (LEDs) are commonplace in TV screens, light bulbs, and other devices. LEDs are crystalline. Electronic clocks in devices of many sorts, microphones and telephones, pickups for guitars, and ultrasound devices in hospitals are based on the piezoelectric effect of a quartz crystal. And roof-top photovoltaic systems use crystalline materials to generate electricity.
It is, perhaps, a stretch for mineralogists to claim credit for modern electronic devises. After all, most of the crystalline materials used in modern electronic applications today are synthetic. Yet they all have natural analogs. And, in large part, it was the study of those analogs and other investigations of mineral crystals that were the start that led to today’s industries based on crystal technology.
1.2 Definition of a Mineral
The word mineral means different things to different people. In ancient times, people divided all things on Earth into the animal, vegetable, or mineral kingdoms, so a mineral was any natural inorganic substance. Today, dieticians use the term to refer to nutritional elements such as calcium, iron, or sodium, while miners often use it for anything they can take out of the ground—including coal, sand, or gravel. Mineralogists have their own definition.
1.2.1 Traditional Definition
James D. Dana (1813-1895), who developed the first widely used mineral classification system (which forms the basis of the one used today), defined a mineral as “. . . a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties.”
Dana’s definition was arguably the first, but more recent definitions have been quite similar. Some sticking points today are whether minerals can be wholly or partly anthropogenic (created by human activity, such as crystalline compounds in coal ash), whether minerals may have biological origins, and whether some mineral-like, non-crystalline materials can be considered minerals.
The International Mineralogical Association (IMA) is the most widely recognized authority on mineral names and nomenclature. The IMA has developed specific criteria that must apply for a substance to be considered a mineral. Today, the IMA list of approved minerals contains 6,000-7,000 entries (http://cnmnc.main.jp/). Many of the names, however, were “grandfathered-in.” They were approved for historical reasons but do not meet the current IMA criteria. And other names, such as olivine and biotite, that are commonly used to refer to minerals, are not on the list because they do not have specific compositions. (Olivine is a general name given to minerals that are primarily solutions of fayalite and forsterite, and biotite refers to micas with compositions between annite and phlogopite.)
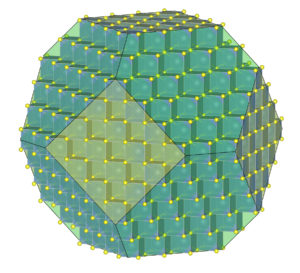
The IMA definition requires that minerals have highly ordered structures, which means that minerals must be crystalline. This is a key thing that separates minerals from many other solid materials. The drawing in Figure 1.6 shows calcium and fluorine ions in a crystal of fluorite. The fluorine ions (small and yellow) are at the corners of cubes called unit cells; the calcium ions (larger and lighter colored) are in the centers of the unit cells.
The model crystal in Figure 1.6 contains a few hundred unit cells and the crystal faces have “steps” in them. A real fluorite crystal, if large enough to see, will contain more than 1020 unit cells. If the crystal contains no defects, the unit cells stack together in an orderly and repetitive way – as in this model drawing – but cells are so small that crystal faces are smooth.
For more discussion about what constitutes a mineral, try this video:
blank▶️ Video 1-1: What is a mineral? (9 minutes)
1.2.2 Matter and Minerals
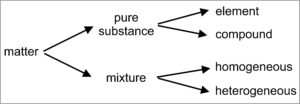
All matter can be classified as being either an essentially pure substance or a mixture (Figure 1.7). Mixtures, in contrast with pure substances, are made of two or more substances that can differ in composition or properties, and that can be separated from each other. Some mixtures are homogeneous (like a drink in which gin and tonic are completely mixed) and some are heterogeneous (like a gin and tonic with ice cubes floating in it), but they can always be separated into individual and different substances using some sort of physical process. Most rocks are examples of mixtures – they can be separated into individual mineral components that have different properties.

Minerals and other pure substances have invariant compositions and distinctive properties. This means that although we can often divide a pure substance into portions, all portions are equivalent and have the same overall composition and properties. For example, we can take a large piece of quartz, perhaps any of the quartz crystals in Figure 1.8, and break it into smaller pieces – the pieces will all still be quartz, the atoms in every piece will all be arranged the same way, and all pieces will have the same hardness and other properties.
Pure substances, including minerals, may be made of a single element, for example argon gas (Ar) or the mineral copper (Cu). Or, they may be compounds made of two or more elements in fixed proportions. Quartz (SiO2), consisting of two elements (silicon and oxygen), is a very common natural compound. Other minerals may consist of more than two elements, and some contain many elements.

Some sand is made of a single mineral, perhaps quartz or gypsum, but most sand is a mix of materials. The sand seen in Figure 1.9 is a good example of a geological mixture. Most sand is made predominantly of quartz, sometimes also containing heavy minerals such as magnetite. This sand is different. It contains several minerals (1 mm sized green olivine grains are most dominant), some black volcanic glass, and two kinds of shell fragments (one white and one with pinkish stripes). We can, in principle, separate all the different components from each other. (Although it would probably be quite tedious.) Note that, except for the shell fragments, all the grains in this sand are very well rounded. This is because they were abraded while being transported before deposition.
● Box 1-1 The Standard Definition of a MineralMinerals are: |
So, although the definition of a mineral is sometimes debated today, for most purposes it is sufficient to say that minerals are natural crystalline solids that generally form by geological processes. They must also be elements or compounds with a well-defined chemical composition that can be described by a chemical formula. The few exceptions to these criteria are dealt with on a case-by-case basis by the International Mineralogical Association (IMA) and others who are tasked with keeping track of all approved minerals.
1.2.3 Minerals and Mineral Varieties


An individual mineral species, such as calcite, is defined by its unique chemical and physical properties. All calcite is mostly CaCO3, with atoms arranged in the same way, no matter the size or shape of the sample. However, calcite, like many other minerals, has more than one named variety, based on crystal shape, composition, color, occurrence, or other things. Dogtooth spar – an example is in Figure 1.10 – is a distinctive variety of calcite found in some caves, and Iceland spar (Figure 1.11) is a clear variety typically in cleavable rhomb shapes.
Gemologists commonly name mineral varieties based on color. Figures 1.12, 1.13, and 1.14, below, show three varieties of beryl. Beryl is called aquamarine if it has a light blue color, emerald if it is green, and morganite if it is pink. The different colors stem from very small compositional differences; all beryl is essentially Be3Al2Si6O18. Aquamarine, however, contains small amounts of Fe2+, emerald contains small amounts of chromium (Cr3+) and vanadium (V3+), and morganite contains Mn2+. Even small amounts of these transition metal ions can give minerals strong coloration.



1.2.4 Synthetic Minerals and Simulants

According to the definition given above, minerals must form by natural geological processes. However, synthetic minerals are commonly made for industrial or commercial use. For example, zeolites sold as health products, such as the product shown here, are generally synthetic. And, because zeolites are natural sieves and sorbents, synthetic zeolites are used in water softeners and in chemical manufacturing processes. The synthetic zeolites are fundamentally the same as naturally occurring minerals – they share many of the same properties – but are prized for engineering and industry because they have purer compositions and more consistent physical properties.
| Gem |
Mineral |
| ruby | corundum |
| sapphire | corundum |
| diamond | diamond |
| emerald | beryl |
| aquamarine | beryl |
| amethyst | quartz |
| citrine | quartz |
| alexandrite | chrysoberyl |
| moonstone | K-feldspar |
| topaz | topaz |
| zircon | zircon |
| opal | opal |
And, we make many synthetic gems, too, today; we have been doing so for almost 150 years. Some of these “fake” gems are beautiful and valuable. Common ones include ruby, sapphire, diamond, emerald, amethyst, citrine, and alexandrite. The table on the right lists these gemstones and others that may be synthesized, along with and their mineral equivalents. Some of the gems have the same name as their mineral equals; but many do not. Ruby and sapphire are both varieties of corundum, amethyst and citrine are varieties of quartz, emerald is a variety of beryl, and alexandrite is a variety of chrysoberyl.
Manufacturers also make other gem-like synthetic crystalline materials that have no natural mineral analogs. They are commonly called simulants and include, among others, forms of cubic zirconia, titanium oxide, and strontium titanate that look something like diamonds. Synthetic gemstones and simulants are often of high quality and mimic natural minerals well. And they may be unflawed and more perfectly formed than natural gems. Distinguishing synthetics from real minerals can be quite challenging. For this reason, some people make no distinction between synthetic and natural minerals.


The two photos of red stones (left) are natural corundum from Tanzania and synthetic corundum grown in a laboratory. The synthetic corundum was faceted (ground) to give it flat faces and sparkle. We call red varieties of corundum, like the corundum shown, ruby. Corundum of any other color is called sapphire. Blue sapphires are most common, but pink, yellow, and other colors exist.


The blue stone in Figure 1.19 is natural topaz from Sri Lanka. Strong aquamarine blue is a rare color for topaz, which is typically clear or light colored. So, most commercial blue topaz is treated to create its blue color. The faceted stones in Figure 1.18 are natural topaz that has been irradiated. Note that the natural topaz, and the natural corundum above, contain smooth crystal faces that formed as the mineral crystallized. The faceted topaz and corundum stones have planar surfaces created by grinding and polishing
Although the compositions and crystallinity of synthetic corundum and other synthetic minerals are nearly identical to natural specimens, synthetics are not considered true minerals. Mineralogical purists would also argue that minerals that have been altered, such as the topaz crystals in Figure 1.18, are no longer true minerals.
1.2.4 Crystalline and Non-Crystalline Mineral Materials
Later chapters will discuss crystals and crystallinity in more detail. For now, it is sufficient to know that crystalline means “having an orderly and repetitive atomic structure.” The definition of a mineral given above includes all crystalline materials made by geological processes. Because minerals are crystalline, they must be solids. However, the International Mineralogical Association (IMA) has granted a special exception to mercury.

Mercury, although liquid under Earth-surface conditions, is considered a mineral because it is a naturally occurring native element like copper, gold, silver, and several others (that are solids except at high temperature). Figure 1.20 shows red, partially cubic crystals of the mineral cinnabar (mercury sulfide) from a famous mineral locale near Almaden, south of Madrid in Spain. Also seen are shiny droplets of silver-colored liquid mercury. Both cinnabar and mercury are minerals. Note that water, the most common natural liquid at Earth’s surface is not a mineral, but ice – crystalline H2O – meets every requirement of the definition and is.

Some natural geological substances appear a lot like minerals but are not crystalline. Instead, they are amorphous solids, meaning they contain a random arrangement of atoms. With a few exceptions (e.g., the copper carbonate georgeite), such materials are not accepted as minerals by the IMA and are generally called mineraloids. Examples of mineraloids include obsidian and several other varieties of natural volcanic glass. This photo shows black obsidian in Iceland. The material surrounding the obsidian is made of scoria and other volcanic debris. Obsidian and other volcanic glasses form when lava cools so quickly that atoms do not have time to arrange themselves in an orderly and repetitive way.

Opal contains silicon and oxygen. It has about the same composition as quartz but contains up to 10% water. On an atomic scale, opal is not crystalline; it comprises spheres of silica (SiO2), 150-300 nanometers in diameter, arranged in a random pattern. The spheres cause light refraction and give some opal a beautiful appearance with rainbow-like colors. If the colors change when the sample is viewed at different angles, we call the property a play of colors. The sample in Figure 1.22, from Ethiopia, displays a beautiful example of play of colors. Because of its play of colors, and because it is very hard, opal is often a much-prized gemstone. The most common kind of opal is like the opal shown, with a rainbow of pastel hues. Black opal is darker colored in blue, gray and green. Fire opals contain brilliant sparks of red, yellow and orange. Like mercury, the IMA has granted opal the right to be called a mineral even if it is not crystalline.
Agate and chalcedony, two varieties of microcrystalline silica (SiO2) related to opal, have the same composition as quartz, but strictly speaking are not minerals because they are not crystalline. They also, typically, consist of more than one mineral. Some mineralogists, however consider agate and chalcedony to be textural varieties of quartz.
1.2.5 Biominerals
Some living organisms produce crystalline materials through a process called biomineralization. The result may be shells or skeletal parts or simply the hardening of soft tissues. Mineralogists have identified more than 60 different biominerals created by animals, plants, fungi, and smaller organisms; three examples are shown below in Figures 1.23 through 1.25.
  |
Organisms that produce biominerals in shells, teeth, skeletons, or bones have existed for nearly 600 million years. Their hard parts are typically composed of organic equivalents of the minerals calcite (calcium carbonate – that makes up the shell shown) and apatite (calcium phosphate – that makes up the teeth shown).
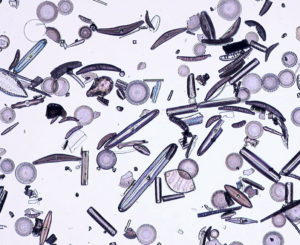
Biogenic processes produce other mineral equivalents too. For example, diatoms (like those seen in Figure 1.25), algae and sponges create structures made of various forms of silica – which is sometimes crystalline (and sometimes amorphous). And, bacteria deposit iron, copper and gold minerals, including iron oxides/hydroxides such as magnetite, goethite, and limonite. Some marine organisms produce aragonite, which is normally only stable under high pressures deep within Earth.
The IMA definition says that a substance is not considered a mineral if it was formed entirely by an organic process. Sometimes, however it is difficult to make this distinction. In some limestones, for example, it is impossible to determine whether a mineral grain precipitated from water (inorganic) or is a biomineral (organic). And, today, some people consider biominerals to be the same as any other minerals. They note the many different kinds of crystalline biogenic substances that, in many cases, are nearly identical to naturally occurring minerals. Additionally, the IMA makes exceptions for some substances formed from organic material by geological processes, such as minerals that crystallize from organic matter in shales.
1.2.6 Anthropogenic Minerals

Crystalline materials that derive from human-produced materials or actions, but meet the definition of a mineral in other ways, are sometimes considered minerals – but generally not. For example, the rust that forms on our cars is not considered a mineral (although the mineral goethite has nearly the same composition and properties). And simonkolleite, a hydrated zinc chloride mineral (Figure 1.26), has been found in some smelter slag but nowhere else.
In 2017 Robert Hazen identified 208 distinct mineral species – all approved by the IMA – that only exist because of human activities and materials. There are no natural analogs. Most of the 208 derive from mining activities – perhaps as scaling on mine walls, as new compounds created in mine dumps, or as precipitates at high temperature in smelters or at low temperature from mine waters. A few developed by alteration of human materials, for example due to weathering of ancient lead or bronze artifacts. In 1998, the IMA decided that, going forward, no substances derived from human-created materials or activities could be called a mineral. But, they have made a few special exceptions since then and have “grandfathered in” many previously identified mineral species.

The photo seen in Figure 1.27 shows red-orange martyite, a zinc-vanadium hydrated mineral that precipitated from mine waters flowing from the Blue Cap Mine near Moab, Utah. This mineral would not exist if it were not for mine waste waters flowing through tailings piles. It was officially approved as a mineral in 2007 and retains that honor today.
1.3 Elements, Minerals, and Rocks
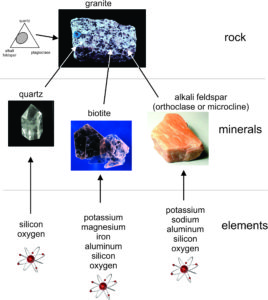
This figure (1.28) shows the relationships between elements (bottom), minerals (center), and rocks (top). Elements, singly or in combination, make up minerals. For example, some of the most common elements in Earth’s crust make up the minerals quartz, alkali-feldspar, and biotite. Minerals, singly or in combination, make up rocks. For example, subequal amounts of quartz and alkali-feldspar, sometimes with biotite and plagioclase, make up granite, a common crustal igneous rock (triangular diagram at the top of the figure).
All minerals, like all materials, consist of one or more elements, the building blocks of all matter. Some minerals, diamond for example, contain a single element (carbon). Others contain many elements. Some minerals have compositions that vary little in nature. Quartz for example is always close to 100% silicon and oxygen in the atomic ratio 1:2. Other minerals incorporate elemental substitutions, so their compositions may vary a great deal from sample to sample. Biotite, for example, always contains potassium, magnesium, iron, aluminum, silicon, and oxygen. It generally also contains lesser amounts of manganese, sodium, titanium, and many other elements, so natural biotite compositions are quite variable.
Rocks are aggregates of one or more minerals, mineraloids, and organic components. Rocks may form when minerals grow (crystallize) together, forming a crystalline rock, such as the granite shown above. They also can form when loose grains are cemented together, forming a clastic rock, such as sandstone. Crystalline rocks may form from a magma (e.g., granite), may form by metamorphism (e.g., gneiss), or may form by precipitation from water (e.g., gypsum). Most clastic rocks form from consolidated sediments, but some form by volcanic processes.

Some rocks contain only one kind of mineral. Limestone (rock), for example, is often pure calcite (mineral). Anorthosite (rock) is made mostly or entirely of plagioclase (mineral). Quartzite (rock) may be made only of quartz (mineral). Dunite (Figure 1.29), an igneous rock that crystallizes from magma, is often nearly 100% olivine (mineral). The dunite shown in the photo also contains a few dark grains of chromite (mineral).
Limestone, anorthosite, quartzite, dunite, and most other rocks contain minerals. Some, less common rocks, however contain virtually no minerals. Examples include as pumice, which is almost entirely volcanic glass, and coal, which is mostly organic materials.
1.4 Classifying Minerals
Chemical formulas form the basis for the standard mineral classification system used today. It is generally called the Dana System of Mineralogy and was created in the mid-19th century by American mineralogist, James Dwight Dana (the same mineralogist who produced the definition of a mineral discussed above in Section 1.2.1). At the largest scale, the Dana System divides minerals into classes based on chemistry. The table below lists the most important classes and key characteristics of their formulas. For example, all silicate minerals contain Si and O. Halides contain Cl, F, Br , or I. Hydroxides contain OH, carbonates contain CO3, and so forth. Oxide minerals are those that contain oxygen but do not belong to one of the other classes. The column on the right lists sample minerals (and formula) for each class.
|
Mineral Classes |
||
| class | key elements or molecules | example mineral |
| silicates | Si and O | quartz – SiO2 |
| halides | Cl, F, Br , I | halite – NaCl |
| hydroxides | (OH) | gibbsite – Al(OH)3 |
| carbonates | (CO3) | calcite – CaCO3 |
| nitrates | (NO3) | nitratite – NaNO3 |
| borates | (BO3) or (BO4) | sinhalite – MgAlBO4 |
| sulfates | (SO4) | gypsum – CaSO4∙2H2O |
| chromates | (CrO4) | crocoite – PbCrO4 |
| tungstates | (WO4) | scheelite – CaWO4 |
| molybdates | (MoO4) | wulfenite – PbMoO4 |
| phosphates | (PO4) | apatite – Ca5(PO4)3(OH,F,Cl) |
| arsenates | (AsO4) | scorodite – FeAsO4∙4H2O |
| vanadate | (VO4) | vanadinite – Pb5(VO4)3Cl |
| oxides | O | corundum – Al2O3 |
| native elements | single elements | copper – Cu |
| sulfides | S | pyrite – FeS2 |
| sulfosalts | As, Sb | niccolite – NiAs |
The Dana System has been modified and fine-tuned several times since its inception and large classes have been subdivided. But the fundamental classes have not changed. The beauty of this system is that it is relatively straightforward for most minerals and requires no information other than chemical formula. A small number of minerals belong to more than one class, but for the most part classification is unambiguous.


Dividing minerals into classes based on formulas is convenient. Furthermore, minerals within a single class are often found together. Besides these pluses, however, the classification scheme makes sense in another important way. The different classes listed in the table above are distinguished by the anions or anionic groups they contain. This means that, within each class, the type of structure and bonding are somewhat similar. Consequently minerals within a class often have similar physical properties, making the classes useful in mineral identification.
Suppose we tried classifying minerals based on the cations in their formulas? Consider, for example, two Fe-minerals: the carbonate mineral siderite (FeCO3) and the sulfide mineral pyrite (FeS2), pictured here. Although both contain Fe2+, they have few properties in common. The siderite (Figure 1.30) is in the form of “roses” and is on top of quartz and chalcopyrite. The pyrite (Figure 1.31) is in golden cubes surrounded by quartz grains in sandstone.
The IMA officially recognizes more than 5,500 minerals (5,650 as of December, 2020). About half are named after people, the rest mostly have names that refer to discovery locations, chemical compositions, or to mineral properties. Most common minerals belong to the silicate, oxide, hydroxide, or sulfide and sulfosalt classes. Oxides and hydroxides together account for about 500 species. Sulfides and sulfosalts also account for about 500 species. The silicate class contains the largest number of minerals, with more than 800 known. In contrast, the native element class contains just a handful of members.
1.4.1 Subclasses, Groups, and Smaller Divisions
The mineral classes listed above are conveniently subdivided into subclasses, groups, series, or subgroups until finally reaching individual species. For example, consider minerals of the silicate class. Silicates make up more than 99% of the minerals found in igneous rocks and account for more than 90% of the Earth’s crust and mantle. Because the silicate class contains many important minerals, we divide it into subclasses.
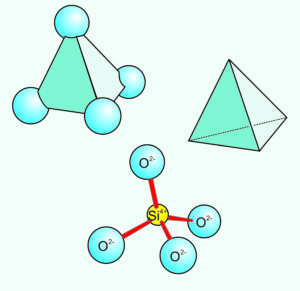
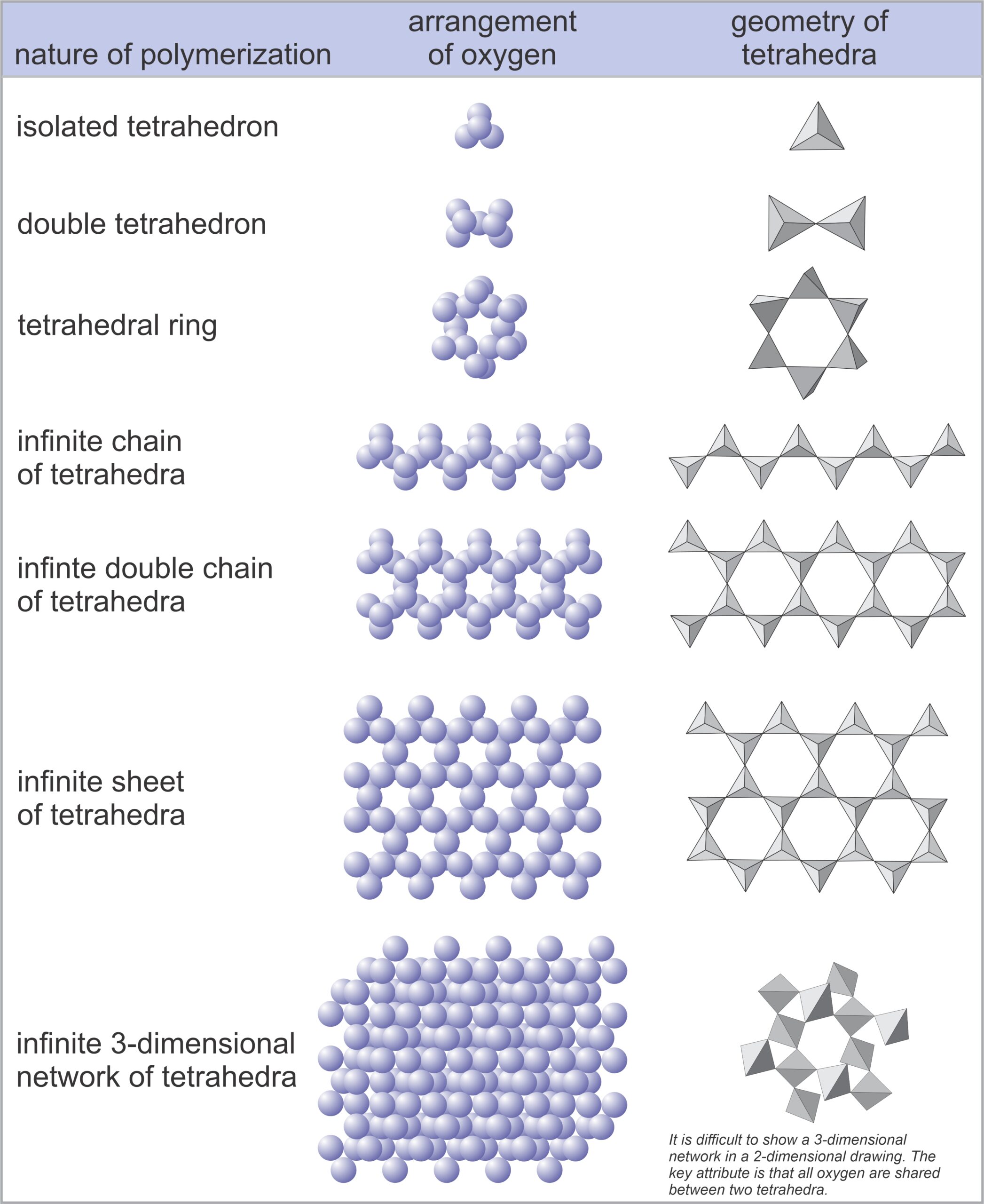
In silicates, except for very rare high-pressure minerals, all silicon atoms are surrounded by four oxygen atoms – arranged in the form of a tetrahedron, a pyramid shape with four identical faces. We can depict the tetrahedra in several ways; Figure 1.32 shows several examples. In mineral structures, these tetrahedra may share oxygen atoms to form chains, sheets, or three-dimensional networks. The linking forms atomic structures called polymers. We name the silicate subclasses according to how silicon tetrahedra are linked (polymerized). Figure 1.33 below shows the possibilities.
1.4.2 An Example: Pyroxenes
Let’s consider the pyroxene group of minerals. All pyroxenes have the general formula ABT2O6. The A and B atoms may be the same or different but are typically Fe, Mg, Ca, Mn, and sometimes Na. T atoms are mostly Si but sometimes up to half Al. The chart below shows how pyroxenes are classified into class, subclass, group, series or subgroup, and species.
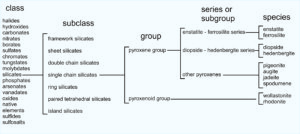

As seen in the chart, pyroxenes belong to the silicate class and single chain silicate subclass. Single chain silicates include minerals of the pyroxene group and minerals of the pyroxenoid group. The groups contain specific species such as enstatite (Mg2Si2O6), ferrosilite (Fe2Si2O6), diopside (CaMgSi2O6), hedenbergite (CaFeSi2O6), and others listed in the far right column of the chart above.
The pyroxene group also contains series, for example the diopside-hedenbergite series, and the enstatite-ferrosilite series. Series define a range of possible mineral compositions between two mineral species. Thus, from general to specific, pyroxenes belong to a mineral class, subclass, group, series, and then are identified as individual species. These relationships hold true for other mineral groups as well. The photo in Figure 1.35 shows light green diopside (a pyroxene) with red garnet and dark purple clinochlore in the background.

Although not shown in the chart above, we divide individual mineral species into varieties based on specific characteristics such as color or crystal shapes. For example, varieties of diopside include chrome diopside (the emerald green chrome containing variety seen in Figure 1.36), dekalbite (diopside that contains no impurities at all), and malacolite (a light colored or white variety of diopside that is usually fluorescent).
● Box 1-2 Chemical Formulas of MineralsThroughout this book, we follow standard chemical conventions when we write mineral formulas. We list elements with subscripts to indicate the relative numbers of atoms present. We list cations (positively charged ions) before anions (negatively charged ions) and molecular anionic species, with the largest cations coming first. The following are some examples of formulas following these rules: Subscripts outside parentheses apply to everything within if no commas are present. The formula unit of marialite, for example, indicates that 4 Na, 3 Al, 9 Si, 24 O, and 1 Cl are in one formula of marialite. Commas show an either-or situation. In one formula of clinohumite, for example, there are two atoms of OH or of F, or of the two combined. If we had omitted the comma, it would mean that there were both 2 OH and 2 F per formula. Elements separated by commas, then, can be thought of as substituting for each other. For example, montmorillonite may contain either Al or Mg, or both; olivine may contain either Fe or Mg, or both. Parentheses surround anionic groups such as (SiO4) or (CO3) when it helps with clarity. In clinohumite, parentheses around (SiO4) emphasize clinohumite’s chemical similarity to forsterite and other olivines, all of which have (SiO4) in their formulas. In montmorillonite, the (Si4O10) is in parentheses to emphasize that the structure is that of a sheet silicate, many of which have (Si4O10) in their formula. Loosely bonded interstitial components (such as Cl in marialite, or OH and F in clinohumite) are on the right in the formulas. We indicate loosely bonded H2O, often called nonstructural water, by a dot preceding nH2O at the far right in a formula. The n (instead of an integer) in the formula for “clay” means that an unknown or variable amount of nonstructural water is present. Natrolite, a zeolite, has H2O in holes in its structure. When completely hydrated, there are two moles of H2O for each Na2Al2Si3O10 formula unit. When useful, we use superscripts to show ionic charge: (OH)– is the hydroxyl radical, which has a charge of -1. Similarly (SiO4)4- indicates an Si atom bonded to 4 O, with a net charge of -4. Sometimes showing coordination number (the number of bonds to an atom) is useful. We do this with superscript roman numerals; they are discussed later. |
blank line
●Figure CreditsUncredited graphics/photos came from the authors and other primary contributors to this book. 1.1 Blue cavansite on top of silvery heulandite, Géry Parent, Wikimedia Commons Video 1-1 What is a Mineral? Keith Purtirka, YouTube |