7 Sedimentary Minerals and Sedimentary Rocks
KEY CONCEPTS
- Weathering involves the decomposition and breaking apart of rocks at Earth’s surface.
- Weathering produces solid clastic material and dissolved chemical material.
- Products of weathering may be transported and deposited to produce clastic or chemical sediments.
- Sediments of all sorts may be lithified to become rocks.
- Clastic material typically comprises quartz and clays; less commonly other minerals.
- Minerals produced by chemical precipitation include clays, carbonates, sulfates, halides, zeolites, and chert.
- We name clastic sedimentary rocks based primarily on clast size.
- We name chemical sedimentary rocks based primarily on composition.
7.1 Weathering
Sediments and sedimentary minerals are products of weathering that involves the physical degradation and chemical alteration of rocks at the Earth’s surface. It is caused by chemical reactions involving air, water, salt, or acid, by freezing and thawing, and by plants and animals. During weathering, once solid rock breaks apart and original primary minerals may decompose. The products include smaller pieces of rock, individual mineral grains, dissolved material that is carried away, and sometimes new secondary minerals.
7.1.1 Two Kinds of Weathering
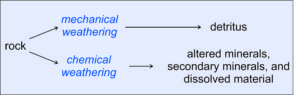
Figure 7.2 shows the two parallel processes that may occur during weathering. An original source rock (igneous, metamorphic, or sedimentary) is exposed to forces that cause weathering. The weathering forces may be mechanical (due, for example, to forces applied by water, wind, or glaciers) or chemical (for example, dissolution by water). Often the two kinds of weathering work together. And, these processes are selective. Some minerals dissolve or react and disappear faster than other minerals. Some rocks are harder and do not break apart as easily as other rocks. Over long times – geological times – chemical weathering has a much greater effect than mechanical weathering. Even apparently dry climates have enough water to promote chemical weathering on exposed surfaces, although the weathering rate may be slow.
Mechanical weathering breaks large or solid material into smaller pieces. The products produced vary greatly; we call them collectively clasts (from klastos, the Greek word meaning “broken”). Clastic material, also called detritus or detrital material, may be fine grains of individual minerals, or it may be lithic fragments (rock fragments) composed of multiple minerals.

The photo seen here (Figure 7.3) shows boulders on a talus slope that were produced by mechanical weathering (mostly freezing and thawing) in Yellowstone National Park. Freezing, thawing, and the action of ice created large blocky pieces of what was originally solid bedrock. Even apparently solid granites or other rocks can be broken apart this way. Talus slopes are examples of very coarse sediment. More commonly, mechanical weathering produces smaller rock fragments, or sand, or silt composed of individual mineral grains.

After chemical weathering, leftover rock may have a dissolved or eroded appearance, such as the sandstone seen in Figure 7.4. This sandstone has weathered to obtain a honeycomb texture, typical of sandstone in which the cementation of grains is not uniform.
Weathering textures are not unique to sandstone. After chemical weathering, outcrops of many sorts often become rounded or pitted. The limestone outcrop shown in Figure 7.5, for example, shows signs of dissolution and chemical weathering. The surfaces are a dull chalky white and the corners are all rounded. Figure 4.24 (Chapter 4) shows another example of chemical weathering, a weathering rind on sandstone.

Minor chemical weathering can cause minerals to alter, perhaps to discolor or rust. More intense weathering may cause some minerals to disappear. They may dissolve completely in water and be carried away in a hydrolysate (water containing dissolved ions). Often, minerals react to produce secondary minerals that were not present before weathering. Reactions that produce secondary minerals most commonly involve water reacting with previously existing minerals. For example, during chemical weathering, feldspars (common in many igneous rocks) react with water to produce clays and dissolved elements. We call such reactions hydrolysis reactions. Secondary minerals may also form by oxidation reactions when primary minerals react with oxygen in air or water. For example, oxidation of iron-rich olivine or pyroxene commonly produces hematite (Fe2O3).
Mineral matter remaining after chemical weathering often includes original mineral grains that did not decompose. We sometimes call these minerals the residual minerals, or the resistate, because the minerals resisted weathering. Typical resistate minerals include quartz, clay, K-feldspar, garnet, zircon, rutile, or magnetite. After the more easily decomposed minerals break down and disappear, the resistate minerals remain to become sediment.
7.1.2 Results of Weathering
If we examine fresh (unweathered) outcrop in a road cut, rock often appears hard and shiny. Examination with a hand lens reveals that minerals have well-defined boundaries and generally sharp outlines. They may show good cleavage or crystal faces. Minerals may have their normal diagnostic colors: quartz is clear, feldspars are white or pink, muscovite is silvery and sparkly, magnetite appears metallic, and biotite and other mafic minerals appear black.

The picture is not the same if we examine outcrops exposed to weathering for a long time. After weathering, rock and most minerals have a dull or drab appearance. Grain boundaries and cleavages are obscured. Oxidation (rusting) and hydration may produce reddish, yellow, brown, or gray hues. Sometimes a layer of clay or other material coats all surfaces, obscuring diagnostic minerals. The photo shown here (Figure 7.6) shows granite that is being replaced by kaolinite (a clay) during weathering. The once solid crystalline granite is now a dull earthy mass.
Mafic silicates weather to create secondary clay minerals and iron oxides. Feldspars of all sorts weather to become clay minerals and dissolved material. Quartz is usually unchanged by weathering. Calcite weathers by dissolution producing dissolve ions. And aluminous minerals weather to gibbsite or other aluminum hydroxides. The table below lists weathering products for the most common minerals. Clays and limonite (a general term describing for a mix of hydrated Fe-oxides and hydroxides) dominate the list. Quartz and aluminous minerals are quite common too. While creating these secondary minerals, weathering also produces dissolved cations (especially alkalis and alkali earths) and anions, which may have a significant impact on water chemistry and quality.
| Typical Chemical Weathering Products of Common Minerals | ||
| mineral (composition) | residual minerals | dissolved ions |
| halite (Na chloride) | Na+, Cl– | |
| gypsum (hydrated Ca sulfate) | Ca2+, (SO4)2- | |
| calcite (Ca carbonate) | Ca2+, (HCO3)– | |
| quartz (SiO2) | (SiO4)4- | |
| plagioclase (Ca-Na-Al silicate) | clay minerals | Ca2+, Na+, (SiO4)4- |
| alkali feldspar (K-Na-Al silicate) | clay minerals | K+, Na+, (SiO4)4- |
| olivine (Fe-Mg silicate) | limonite, hematite, clay | Mg2+, (SiO4)4- |
| pyroxene (Ca-Fe-Mg silicate) | limonite, hematite, clay | Ca2+, Mg2+, (SiO4)4- |
| amphibole (Ca-Fe-Mg silicate) | limonite, hematite, clay | K+, Mg2+, (SiO4)4- |
| biotite (K-Fe-Mg-Al mica) | limonite, hematite, clay | K+, Mg2+, (SiO4)4- |
| muscovite (K-Al mica) | clay | K+ |
7.1.3 Ease of Weathering
Some minerals break down more easily than others. Geologists can compare weathering rates by looking at minerals in rock outcrops, and by studying the minerals present in sediments of different ages. Goldich (1938) made such observations, publishing what we call Goldich’s Weathering Series. The series ranked the ease with which common igneous minerals break down. Goldich found that minerals that crystallize from a magma at high temperature – minerals relatively poor in silicon and oxygen – are generally less resistant to weathering than those that crystallize at low temperature. Thus, the minerals at the top of Bowen’s reaction series weather most easily and those at the bottom are more resistant to weathering. Iron-magnesium silicates, such as olivine, pyroxene, or amphibole break down relatively easily. Calcic feldspars, and many minerals with high solubilities in water, are also quick to decompose. Quartz, some feldspars, and some non-silicate minerals are relatively resistant to weathering because they contain more bonds, especially Si – O bonds, that do not break easily. It should not be surprising that minerals that characterize high-temperature igneous rocks, or those most often dissolved in water, are the first to decompose under Earth surface conditions where temperature is low and water is abundant.
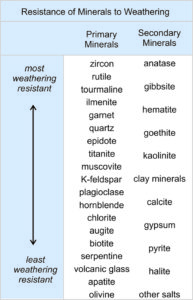 Sedimentologists have made comprehensive lists of the relative ease with which minerals weather. Although there is some variation, the list shown here is typical (from Birkeland 1999). Like primary minerals, secondary minerals can break down and disappear, so this table compares weathering rates for both primary minerals and secondary minerals. Weathering resistance, however, does not necessarily mean that a particular mineral is abundant in weathered materials. Some of the minerals at the top of the list in the table are uncommon compared with others. Zircon, rutile, and tourmaline, for example, are very resistant to weathering but rarely are major components of sediments because they are only minor minerals in most parent rocks. Minerals at the bottom of the list are very unstable when exposed to the elements and, consequently, are absent from all but the youngest sediments.
Sedimentologists have made comprehensive lists of the relative ease with which minerals weather. Although there is some variation, the list shown here is typical (from Birkeland 1999). Like primary minerals, secondary minerals can break down and disappear, so this table compares weathering rates for both primary minerals and secondary minerals. Weathering resistance, however, does not necessarily mean that a particular mineral is abundant in weathered materials. Some of the minerals at the top of the list in the table are uncommon compared with others. Zircon, rutile, and tourmaline, for example, are very resistant to weathering but rarely are major components of sediments because they are only minor minerals in most parent rocks. Minerals at the bottom of the list are very unstable when exposed to the elements and, consequently, are absent from all but the youngest sediments.
After chemical weathering, dissolved material is carried away. Residual minerals and secondary minerals such as clay may remain where they form. For example, prolonged weathering of bedrock can lead to thick layers of reddish soil called laterite in tropical areas (see Box 7-1, below). Laterites vary but are always rich in oxide minerals and clays. Laterites are easily eroded. Over time, erosion by water, gravity, or wind can transport laterite debris, just like any other detrital material, away from its place of origin.
7.2 Siliciclastic Sediments
| The Wentworth Scale for Grain Size |
|
| classification | particle/clast size (diameter in mm) |
| boulder | >256 |
| cobble | 64 to 256 |
| pebble | 4 to 64 |
| gravel or granule | 2 to 4 |
| sand | 1/16 to 2 |
| silt | 1/256 to 1/16 |
| clay | <1/256 |
The term siliciclastic refers to sediments composed mostly of silicate minerals. The most common sedimentary rocks – including shale, sandstone, and conglomerate – form from siliciclastic sediments. Other kinds of sedimentary rocks consist of carbonates (in limestones), iron oxides and hydroxides (such as hematite or goethite in iron formation), or other minerals.
Geologists classify siliciclastic sediments based on grain size. The standard classification system is the Wentworth Scale (see table). Depending on size, clasts may be boulders, cobbles, pebbles, gravel, sand, silt, or clay. The word clay sometimes causes confusion. Sedimentary petrologists use the term to refer to clastic grains smaller than 0.004 mm in longest dimension. They also use the term, however, to refer to minerals of the clay mineral group, no matter the grain size.
Clast sizes vary from fine clay and silt to huge boulders. Small clasts are usually composed of a single mineral, generally quartz or clay. Larger clasts are commonly lithic fragments composed of multiple minerals. The photos below show some examples of sediment containing different kinds and sizes of clasts.
Figure 7.8 is a view of mud along a river in Tasmania. The mud comprises fine grains of silt and clay. Figure 7.9 shows sand from Pfeiffer Beach, California. Quartz dominates most common sand, but the sand seen here contains mostly rosy garnet, and also epidote, zircon, magnetite, spinel, staurolite, and only minor quartz. Figure 7.10 shows centimeter scale pebbles on a beach in Greece. Most of the pebbles are lithic fragments (rock fragments) composed of more than one mineral. Figure 7.11 shows cobbles in a dry river bottom. These cobbles are all lithic fragments. The mineral grains in the Pfeiffer Beach sand are angular, but the clasts in the last two photos have been well rounded by abrasion due to tumbled during transportation by flowing water.
7.3 Transportation, Deposition, and Lithification
7.3.1 Clastic Sedimentation

Flowing water transports clastic material until it reaches a place where it collects, perhaps a river or lake bottom, or maybe a delta. Wind, gravity, and other agents can move clastic material as well. Eventually, sediments are deposited when the forces of gravity overcome those trying to move them.
Large grains may not move far and are deposited first. As the energy of transportation decreases, smaller material is deposited. So, during transportation, sediments commonly become sorted, which means that sediment deposits often have relatively uniform grain size. Thus, for example, coarse material may be deposited near the headwaters of a stream, while only fine material makes it to a delta. Sorting is not ubiquitous. Streambed gravel, for example, may contain a mix of silt, sand, and larger clasts, and glacial deposits often contain a jumble of material of many different sizes. The photo above (Figure 7.12) shows very poorly sorted alluvium (loose, unconsolidated nonmarine sediment deposited by rivers or streams) in Argentina.
After deposition, unconsolidated sediment may, over time, change into a clastic sedimentary rock by the process called lithification (from lithos, the Greek word meaning stone). Lithification involves compaction and cementation of clastic material. Common cementing agents include the minerals quartz, calcite, and hematite.
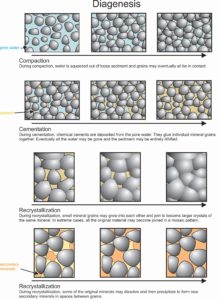
7.13 The processes of diagenesis
Before, during, and after lithification, sedimentary rocks undergo textural or chemical changes due to heating, compaction, or reaction with groundwaters. Biological agents, including small animals or bacteria, also can be important, as can chemical agents brought in by flowing water. We call these changes collectively diagenesis. Figure 7.13 depicts the major steps of diagenesis: compaction, cementation, and recrystallization.
Dissolution and removal of minerals (leaching) and the formation of clay or other minerals are both common during diagenesis. We call any new minerals that form, authigenic minerals. Zeolites, clays, feldspar, pyrite, and quartz can all be authigenic minerals. Although diagenesis creates many authigenic minerals, most are so fine grained that we cannot identify them without X-ray analysis.
Textural changes, including compaction and loss of pore space, are common and are part of diagenesis. Recrystallization, the changing of fine-grained rocks into coarser ones, is another form of diagenetic textural change. During recrystallization, as individual mineral grains grow together, secondary minerals may precipitate in open spaces, and more mineral cements may develop. Consequently, rocks become harder.
Diagenesis is equivalent to a low-temperature, low-pressure form of metamorphism, and the processes of sedimentation, lithification, diagenesis, and low-grade metamorphism form a continuum. Lithification changes unconsolidated sediment into a rock. Cementation by quartz, calcite, or hematite may be part of the lithification process. It also may be considered a diagenetic process. Similarly, the formation of many low-temperature minerals such as zeolites, a normal part of diagenesis, overlaps with the beginnings of metamorphism. Metamorphic petrologists often define the onset of metamorphism by the first occurrence of metamorphic minerals. This definition can be hard to apply because many diagenetic minerals are also metamorphic minerals. Furthermore, laumontite, often considered to be formed at the lowest temperature of all metamorphic minerals, is a zeolite that is hard to distinguish from minerals that form diagenetically.
7.3.2 Chemical Sedimentation

Chemical weathering yields dissolved material that water transports until precipitation of chemical sediment occurs. Several things may cause precipitation; the most common causes are evaporation, changes in temperature or acidity (pH), and biological activity. For example, hot springs deposit a form of calcite called travertine when cooling water becomes oversaturated with CaCO3. This photo (Figure 7.14) shows thick travertine terraces deposited by hot springs in Wyoming.
In freshwater streams or lakes, a pH change due to biological activity may cause precipitation of another form of calcite called marl. In marine settings, many reef-building organisms have shells or skeletons made of organic calcite. So, living organisms are the prcipitating agents. Calcite and other chemical sedimentary minerals, then, precipitate in many ways. In contrast with clastic sediments, chemical sediments usually lithify at the same time they precipitate.

Natural waters contain dissolved minerals, and all minerals are soluble in water to some extent. Halides, many sulfates, and other salts have very high solubilities. Carbonate minerals, including calcite and dolomite, have moderate solubilities. Silicate minerals have relatively low solubilities. If water evaporates, it may become oversaturated in particular minerals and deposit chemical sediments, such as the salt deposits in the photo seen here (Figure 7.15). Precipitation will continue, decreasing concentrations of dissolved material, until the solution and sediments achieve equilibrium. Because of their high solubility, large amounts of evaporation may be necessary before salts such as halite, precipitate. In contrast, carbonate minerals (calcite and dolomite) and silica often precipitate early during evaporation. Silica (SiO2), in the form of chert, is the only silicate mineral that commonly forms a chemical sedimentary rock.
Gypsum (CaSO4•2H2O), anhydrite (CaSO4), halite (NaCl), and sylvite (KCl) consist of common elements. Ggypsum and anhydite have high solubilities; halite and sylvite have even higher solubilities. So, their chemical components are common as dissolved species. As water evaporates, perhaps in a closed inland basin or an isolated sea, these four minerals may precipitate to form thick beds of evaporite minerals. Besides these minerals, many other (less common) minerals also occur in evaporites. Evaporites are found in many parts of the world. Geologists estimate that thick evaporite beds, at some depth, underlie 35% of the United States.

Figure 7.16 shows the Trona Pinnacles in Searles Lake, a southern California playa lake. Some of the pinnacles rise more than 40 m above the lakebed. These pinnacles consist of trona (a hydrated sodium carbonate) that precipitated from briny water. Like all playas, this lake is dry most of the time. But, past flooding and subsequent evaporation produced thick layers of evaporite minerals. More than 25 different minerals are found in the Searles Lake sediments. The list includes sodium and potassium carbonates, sulfates, borates, and halides. Borax (hydrated sodium borate), trona, and several other minerals are profitably mined from Searles Lake sediments today.
Most evaporite minerals are rare at Earth’s surface because they are so soluble that they dissolve away in all but the most arid climates. Gypsum is the most common evaporite in outcrops because it is less soluble than others, and because it forms from any anhydrite exposed at or near Earth’s surface. In the subsurface, massive gypsum and halite beds are common, as are the salt domes found in Texas and other Gulf Coast areas of the United States. Although generally dominated by just a few minerals, many other minerals may be present. In all, petrologists have reported nearly 100 minerals from evaporites. Less than a dozen are common.

Evaporites may be marine deposits associated with evaporation of ocean water. They may also be non-marine, associated with freshwater lakes or other continental waters. For water to become oversaturated, a water body must be somewhat isolated and the evaporation rate must be faster than any water flowing in. This is most common in an arid environment. For example, at various times in the past, the Mediterranean Sea has been cut off from an ocean. Evaporation led to thick salt deposits that lie beneath the Mediterranean today. And today, evaporite minerals are collecting along the shores of the Dead Sea between Jordan and Israel (see Figure 7.1, the opening figure in this chapter), on the shores Utah’s Great Salt Lake (Figure 7.17), and around many other isolated water bodies.
| Some of the Most Common Evaporite Minerals | ||
| mineral group | mineral name | chemical |
| chlorides | halite sylvite bischofite kainite carnallite |
NaCl KCl MgCl2•6H2O KMg(SO4)Cl•3H2O KMgCl3•6H2O |
| sulfates | anhydrite gypsum barite thenardite mirabilite kieserite langbeinite polyhalite kainite epsomite |
CaSO4 CaSO4•2H2O BaSO4 Na2SO4 Na2SO4• 10H2O MgSO4•H2O K2Mg2(SO4)3 K2Ca2Mg(SO4)4•2H2O KMg(SO4)Cl•3H2O MgSO4•7H2O |
| carbonates | dolomite calcite magnesite trona |
CaMg(CO3)2 CaCO3 MgCO3 Na3(HCO3)(CO3)•2H2O |
| borate | borax | Na2B4O7•10H2O |
As ocean water evaporates, minerals precipitate in predictable order from those that are least soluble to those that are most soluble. Calcite is first, followed by gypsum, anhydrite, and then halite. Many other minerals may precipitate in lesser amounts. Continental water contains different dissolved solids than marine water, so continental evaporites contain different minerals than marine evaporites. Water chemistry is also quite variable, so many different minerals are possible.
Continental evaporite deposits may contain halite, gypsum, and anhydrite but also typically have borax, trona, and many other non-marine salts. The table lists some minerals reported from evaporite deposits in North America. It is a long list.
this is a blank line
7.4 Sedimentary Minerals
7.4.1 Common Minerals
The previous chapter discussed silicate minerals common in igneous rocks. In principle, they could all be detrital grains in sediments and sedimentary rocks. In practice, most break down so quickly that they cannot be weathered or transported very much before completely decomposing. Quartz is the most resistant to weathering. It is also a common component of many igneous and metamorphic rocks found at the Earth’s surface. Many minerals weather to produce clays. It is no surprise, therefore, that quartz and clays are the main silicate minerals in most clastic rocks. Feldspars and sometimes muscovite may also be present but are usually subordinate to quartz. They are absent from rocks formed from sediments transported long distances or weathered for long times. Mafic silicate minerals are exceptionally rare in sediments or sedimentary rocks. Besides quartz and clays, other silicates, including zeolites, may occasionally be present. Important non-silicate minerals in clastic rocks include carbonates, sulfates, oxides, halide minerals and occasionally pyrite.
7.4.2 Clay Minerals
| Clay Minerals | ||||
| illite group | K(Al,Mg,Fe)2(Si,Al)4O10(OH)2•nH2O | |||
| smectite group •montmorillonite •nontronite •beidellite •saponite |
(Na,Ca)0.33(Al,Mg)2Si4O10(OH)2•nH2O Na0.3Fe2(Si,Al)4O10(OH)2•nH2O Na0.5Al2(Si3.5Al0.5)O10(OH)2•n(H2O) Ca0.25(Mg,Fe)3(Si,Al)4O10(OH)2•n(H2O) |
|||
| kaolinite group •dickite •halloysite •nacrite |
Al2Si2O5(OH)4 Al2Si2O5(OH)4 Al2Si2O5(OH)4 |
|||
| related minerals •vermiculite •talc •pyrophyllite |
(Mg,Fe)3(Si,Al)4O10(OH)2•4H2O Mg3Si4O10(OH)2 Al2Si4O10(OH)2 |
|||
The clay minerals include many different species; all are sheet silicates. The sheets comprise tetrahedral layers containing mainly Si and Al, and octahedral layers containing mainly Al, Mg, and Fe. Clays generally contain less potassium than micas. Some clays are hydrous, some having as much as 15 to 20 weight percent (wt %) H2O. Their layered structure and the weak bonding between layers give them a characteristic slippery feel when wet.
The table seen here lists the most common clays. The formulas in the box here are only approximate because clays often contain many elemental substitutions. Major differences between the different clay species are the compositions and stacking order of atomic layers.•
Clay minerals account for nearly half the volume of sedimentary rocks. They are usually very fine grained, often less than 1 μm (10-6 m) in size, have complex chemistries, and are structurally variable. This makes identification of individual clay species difficult. X-ray analysis is often necessary to tell them apart. In contrast with quartz and feldspar, clays do not form in igneous and metamorphic environments.
Clays are common in shales and other sedimentary rocks, but the clay species present depends on the sediment sources. Although usually fine grained, clays can form thick beds or layers. They also develop as coatings on other minerals undergoing weathering. These generalizations are true of all clay minerals, but there is a great deal of variety. In part, the variation is due to the low temperatures at which these minerals form. At high temperatures, minerals and mineral structures tend to be simple and ordered. At low temperatures, structures are often more complex or disordered, and many different mineral varieties may form.
The three most important kinds of clays are illite, montmorillonite, and the clays of the kaolinite group. Figures 7.18, 7.19, and 7.20 show examples of each, but these minerals have many different appearances. Kaolinites, also called kandites, vary less in composition and structure than other clays, although several kaolinite polymorphs (dickite, halloysite, nacrite) are known. Kaolinite is the principal clay used to make ceramic ware because it remains white when fired in a kiln. Illite is quite similar to muscovite in some ways, but contains more Si and less K.
Montmorillonite, which belongs to the smectite group, can take up extra water or other fluids between layers of atoms. In the process the clay expands. So, we sometimes call montmorillonite and other clays of the smectite group expandable or swelling clays. Because they absorb liquids so well, gas station operators use them to clean up spilled oil, and homeowners use them as kitty litter. They are the major components of earthy material called bentonite, sometimes prized for its water-absorbing and cation-exchange properties. Vermiculite, another clay of the smectite group, is often used to lighten up potting soil. Montmorillonite dominates modern clay-rich sediments and sedimentary rocks; illite dominates most sedimentary rocks that are older than about 100 million years. Geologists ascribe this development to ongoing diagenesis, to variations in tectonic activity resulting in changes in sediment sources, and to changes in biological activity.
is a blank line
this is a blank line.


Talc, a secondary mineral that forms when Mg-silicates such as olivine or pyroxene are altered, and pyrophyllite, an uncommon metamorphic mineral, are often grouped with the clays. Figures 7.22 and 7.23 show examples of each. Both are less variable in their atomic arrangements and composition and contain less H2O than true clays. They are transitional between clays and micas in structure and, when seen in hand specimen or thin section, are typically easier to identify than clays. Talc is very soft and has a diagnostic greasy feel that generally makes identification straightforward. In contrast, pyrophyllite often looks like many other white minerals, unless it has the characteristic splay of crystals seen in the photo above (Figure 7.23).
7.4.3 Carbonate Minerals
| Carbonate Minerals |
|
| Ca-Mg-Fe carbonates •calcite •aragonite •magnesite •siderite •dolomite •ankerite |
CaCO3 CaCO3 MgCO3 FeCO3 CaMg(CO3)2 CaFe(CO3)2 |
| Other •rhodochrosite •smithsonite •cerussite •strontianite •witherite •azurite •malachite •hydromagnesite •natron |
MnCO3 ZnCO3 PbCO3 SrCO3 BaCO3 Cu3(CO3)2(OH)2 Cu2CO3(OH)2 Mg5(CO3)4(OH)2•4H2O Na2CO3∙10(H2O) |
Mineralogists have identified more than 50 different carbonate species; all contain (CO3)2- groups but some contain other anions or anionic groups. The box lists some examples. The most important carbonates are Ca-Mg-Fe carbonates, especially calcite and dolomite, CaCO3 and CaMg(CO3)2, respectively. Less common rhodochrosite, smithsonite, cerussite, strontianite, azurite, and malachite sometimes form spectacular mineral specimens. These minerals are also important ore minerals of manganese, zinc, lead, strontium, and copper. The common carbonates have relatively simple compositions and include no hydroxyl groups or H2O. Some relatively rare species are more complex, however, and examples are at the bottom of the list.
Calcite is the most abundant carbonate mineral. It typically forms by organic processes or precipitation from oversaturated water. It also occurs in caves, where calcite forms stalactites and stalagmites. Calcite and other carbonate minerals also precipitate from hydrothermal waters (warm waters), especially in ore deposits and typically in veins. This is how most azurite and malachite, and some related ore minerals, are created.

Many carbonate minerals can have either an inorganic or an organic origin. Inorganic marine carbonate rocks form when either calcite or aragonite precipitate from ocean water. Marl may form when carbonates precipitate on lake or stream bottoms. In contrast, organic carbonate rocks form when algae, corals, clams and other organisms create structures and shells from CaCO3 dissolved in seawater. This may produce carbonate rocks directly, or residual shells, bones, and other material can accumulate to produce carbonate clastic rocks. The maze coral seen in this photo (Figure 7.24) builds its structure out of calcite.

Both calcite and dolomite are essential minerals in limestones and dolostones (limestones containing dolomite instead of calcite), and may be clasts in other kinds of sedimentary rocks. Some clastic rocks are held together by fine grained carbonate cement. Both calcite and dolomite are found in metamorphic rocks called marbles and in rare igneous rocks called carbonatites. The photo seen here (Figure 7.25) shows a marble made of coarsely crystalline calcite.

Many carbonate minerals are secondary minerals formed during weathering or diagenesis. For example, most dolomite is secondary and forms by reaction of calcite with Mg-rich water during diagenesis. Magnesite, (MgCO3), a related carbonate, forms as an alteration product of mafic and ultramafic rocks. Hydromagnesite (a hydrated equivalent of magnesite) forms by weathering of Mg-rich minerals, including olivine, serpentine, and others. Figure 7.26 shows hydromagnesite that has crystallized on the surface of basalt.
Photos below show examples of coarsely crystalline carbonate minerals. Figure 7.27 contains calcite crystals (Ca-carbonate) on top of siderite (Fe-carbonate). Figure 7.28 shows aragonite, which is a polymorph of calcite. The blue and green specimen in Figure 7.29 contains azurite and malachite. Both are Cu-carbonates and both are copper ore minerals.



The pinkish crystals in Figure 7.30 are rhodochrosite (Mn-carbonate). Rhodochrosite is not always this color, but when it is, the color helps identify it. The azurite, malachite, and rhodochrosite come from a well-known mining district in western Colorado. The green crystals in Figure 7.31 are smithsonite (Zn-carbonate). Like rhodochrosite, smithsonite comes in various colors, but this green color is typical. The last photo (Figure 7.32) shows crystals of dolomite (Ca-Mg carbonate) on top of talc.



Unfortunately, most mineral specimens are not as well formed or beautiful as suggested by the photos above. So, below are three more typical examples of calcite. The calcite specimen on the left (Figure 7.33, below) is massive; it is difficult to pick out distinct crystal shapes. The blue calcite in Figure 7.34 and the translucent calcite in Figure 7.35 are cleavage fragments. Calcite cleaves easily into rhombohedral shapes like the ones seen here, and the flat surfaces are cleavage planes, not crystal faces. Blue calcite is rare but translucent calcite is not. Rhombohedral cleavage fragments are exceptionally common.



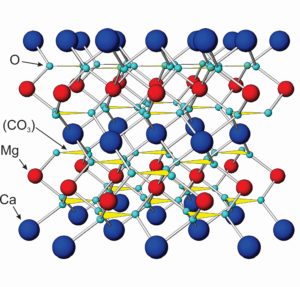
The atomic arrangements in calcite and dolomite, the most common carbonates by far, contain (CO3)2- groups alternating with Ca2+ or Mg2+. In the model of dolomite seen here (Figure 7.36), the blue atoms are calcium, the red ones magnesium, and the small aqua atoms are oxygen. Carbonate (CO3)2- groups are shown as yellow triangles. Other carbonates have similar structures. They all contain divalent anion carbonate groups and divalent cations such as Ca2+, Mg2+, Fe2+, Mn2+, or Zn2+. (Figure 7.36 is not to scale; the size of the carbonate groups is actually larger tham Ca2+ and Mg2+.)
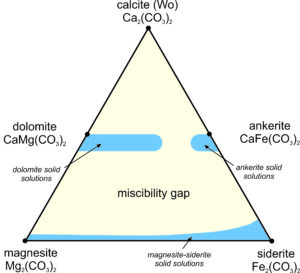
We can plot the compositions of Ca-Mg-Fe carbonates on a triangular diagram (similar to that used for pyroxenes in the previous chapter). Figure 7.37 shows, in blue, the ranges of stable carbonate compositions at low temperature; the stable compositions are limited. A large miscibility gap exists between the Ca-bearing carbonates and the Ca-free ones. It is similar to the gaps between wollastonite, clinopyroxene, and orthopyroxene we have already seen. And, the dolomite-ankerite series does not extend all the way across the diagram. As with the pyroxenes, the size of the miscibility gaps varies with temperature.
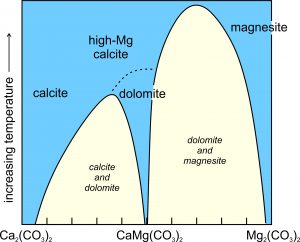
Figure 7.38 shows the simplfied schematic solvus relationships between calcite (CaCO3) and magnesite (MgCO3), equivalent to the left side of the triangle above. (It is simplified because melting and decarbonation reactions occur at high temperatures but are not shown.) We can see that the solvi narrow as temperature increases. At the highest temperatures on the diagram, calcite and high-magnesium calcite are stable. They are both Ca-Mg solid solutions of variable composition. Magnesite, an Mg-Ca solid solution, is stable too. At somewhat lower temperatures, high-Mg calcite changes into dolomite by an order-disorder transformation. The miscibility gaps (in yellow) at low temperature, mean that most carbonate compositions will unmix into calcite and dolomite, or dolomite and magnesite, solid solutions. Calcite may contain some extra magnesium, but the other two minerals will be close to end member composition. Calcite sometimes develops exsolution textures that we can see with a thin section and a petrographic microscope.

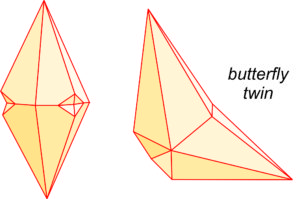
Calcite crystals twin by several different twin laws. The drawings seen here (Figure 7.39) show two common twin appearances. The drawing on the right is called a butterfly twin, and the photo in Figure 7.40 shows a good example of this kind of twinning. Calcite also commonly contains microscopic deformation twins that we can only see with a petrographic microscope.
7.4.4 Sulfate Minerals
| Sulfate Minerals |
|
| gypsum anhydrite barite celestite anglesite |
CaSO4•2H2O CaSO4 BaSO4 SrSO4 PbSO4 |
Mineralogists have described more than 100 sulfate minerals. They fall into two main groups: those that contain water (hydrous sulfates) and those that do not (anhydrous sulfates). Gypsum (CaSO4•2H2O) is the only common hydrous sulfate. Less common species include chalcanthite (CuSO4•5H2O), epsomite (MgSO4•7H2O), and antlerite Cu3SO4(OH)4. Examples of anhydrous sulfates include anhydrite (CaSO4), barite (BaSO4), celestite (SrSO4), and anglesite (PbSO4). Many others are known, but most are rare.
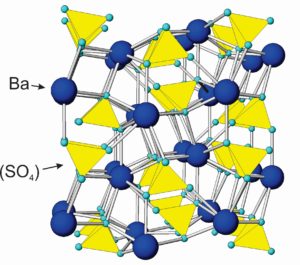
The drawing in Figure 7.41 shows how atoms are arranged in barite (BaSO4). Sulfur atoms bond to four oxygen, creating sulfate tetrahedra with composition (SO4)2-. The tetrahedra are tightly bonded and similar, in some ways, to the (SiO4)4- groups that characterize silicates. Sulfate tetrahedra, however, do not polymerize. Ba2+ cations alternate with anion sulfate and bond to oxygen at tetrahedral corners. The atomic arrangements are the same in the other anhydrous sulfates, with Ca2+, Sr2+, and Pb2+ substituting for Ba2+ in anhydrite, celestite, and anglesite, respectively. Besides the minerals listed in the blue box, at least a dozen other, but rare sulfates exist. Some are listed in the table of evaporite minerals earlier in this chapter.


Gypsum, hydrated calcium sulfate, is the most common sulfate mineral. It is found in thick evaporite deposits, in hydrothermal veins, and as precipitates from surface or subsurface waters, hot springs, or volcanic gases.
Anhydrite, with a composition equivalent to gypsum lacking H2O, is an evaporite mineral found in sedimentary rocks associated with sedimentary basins. It commonly found with gypsum, and alters to gypsum over time. Anhydrite may be in thick layered rocks that also contain halite and carbonate minerals.
Common gypsum is white to grey, and translucent, but just about all colors are known. Normal coarse crystals of gypsum form blades or tabs. If translucent, we call them selenite (Figures 7.42 and 7.43). Selenite commonly twins, sometimes forming characteristic swallow tail or fishtail twins. These two types of twinning are very similar and often not distinguished from each other. Figure 7.43 shows two examples of swallowtail twins. Another example is seen in Figure 4.39 (Chapter 4).


Satin spar is an especially fibrous variety of selenite (Figure 7.44). Satin spar is often opalescent and is sometimes used in jewelry, although it is soft and not very durable. Sometimes gypsum crystals form a flowery cluster called a desert rose (Figure 7.45). The specimen in Figure 7.45 is 47 cm across! In caves gypsum may form gypsum flowers, similar in many ways to desert roses. Alabaster is very fine grained gypsum that people sometimes carve or polish for building stone or for art.

The spectacular photo in Figure 7.46 shows a geologist in Mexico’s Cueva de los Cristales (Cave of Crystals). The cave contains some of the largest mineral crystals in the world – up to 12 meters long. These crystals are selenite, the translucent form of gypsum. Gypsum is moderately water-soluble, so it is one of a relatively small number of common minerals that precipitate from natural water, often redissolve, and later reprecipitate somewhere else. Most natural gypsum crystals are centimeters in size or smaller. However, extremely large crystals, such as those shown in the photo above, exist in several places around the world.


Barite, barium sulfate, commonly occurs in hydrothermal deposits with copper, lead, and zinc minerals; it is often associated with anglesite and celestite. Barite is also found in hot spring deposits, and in some evaporite deposits. The cluster of clear to light-blue bladed crystals in Figure 7.48 is typical for barite. And, barite sometimes occurs as concretions in sediments and sedimentary rocks, and sometimes as desert roses (Figure 7.49), similar to desert roses made of gypsum.


Celestite (also called celestine), strontium sulfate, most often occurs in sedimentary rocks associated with gypsum, anhydrite, sulfur, or halite. Commonly it has a diagnostic light blue color as seen in the photo here (Figure 7.50). Celestite is the most common strontium mineral. Strontianite (strontium carbonate) is the only other one of significance.
Some sulfates are common as minor, and rarely major, minerals in ore deposits – typically as replacements for primary sulfides. Anglesite (PbSO4), for example, forms during weathering or alteration as a replacement for galena (PbS). The specimen seen in Figure 7.51 contains light brownish anglesite that appears to have grown from the silver-gray galena.
7.4.5 Halides
| Halide Minerals |
|
| halite sylvite fluorite |
NaCl KCl CaF2 |
The halide group consists of minerals containing a halogen element, generally chlorine or fluorine, as an essential anion. Although many halides exist, only halite and sylvite are common in sedimentary rocks; they are quite rare in rocks of other sorts. Halite is typically found as rock salt in massive salt beds, often occurring with other evaporite minerals such as gypsum or anhydrite, and sometimes with sulfur. Sylvite is much less common than halite. When found, however, it is usually associated with halite. Fluorite, most commonly, is a hydrothermal mineral found with lead, zinc, and other metal ore minerals. Less commonly, it is found in vugs or fractures in limestone or dolostone. Other halides are rare and have more complex chemistries.


The photos seen here show crystals of halite (Figure 7.52) and fluorite (Figure 7.53). Sylvite crystals commonly look the same as the halite crystals here. When euhedral, both minerals form cubic crystals, a reflection of the internal order of their atoms. The most common fluorite is purple, but just about any color is possible for this mineral.
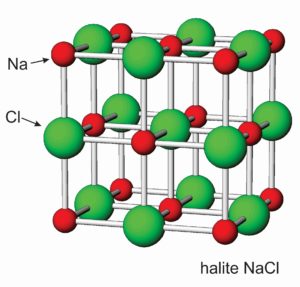
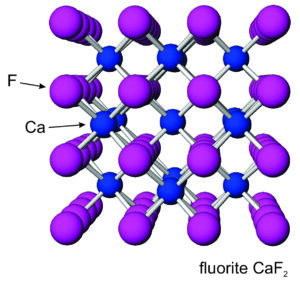
Figures 7.54 and 7.55 show atomic arrangements in halite and fluorite. In halide minerals, anions and cations alternate and bonds are nearly entirely ionic. In halite (or sylvite), Na+ (or K+) and Cl– alternate in a cubic three-dimensional arrangement. In fluorite, the arrangement of atoms is also cubic, but two F– are present for every Ca2+.
7.4.6 Zeolites

This photograph (Figure 7.56) shows orange chabazite crystals on top of heulandite. Both of these are zeolite minerals that occur most commonly in openings within basalt, and less commonly in metamorphic rocks or hydrothermal veins. This sample comes from a classic locality near Wasson Bluff, on the shore of the Bay of Fundy, Nova Scotia, where many outcrops of 200-million year old basalt contain cavities filled with spectacular mineral specimens.
Although zeolites are best known for their occurrences in vugs and other open cavities in volcanic rocks, large volumes are found in volcanic ashes and saline lake deposits. Zeolites also may be present as products of diagenesis or low-grade metamorphism in sediments and rocks.
| Zeolite Minerals |
|
| natrolite analcime laumontite chabazite clinoptilolite heulandite stilbite sodalite |
Na2Al2Si3O10∙2H2O NaAlSi2O6·H2O CaAl2Si4O12∙4H2O CaAl2Si4O12∙6H2O (Na,K)Al2Si7O18∙6H2O CaAl2Si7O18∙6H2O CaAl2Si7O18∙7H2O Na3Al3Si3O12∙NaCl |
The zeolite group includes more than 40 minerals. They are, essentially, hydrated feldspars and are framework silicates containing open cavities that can hold loosely bonded large cations and water.
The compositions of zeolites seem highly variable, but follow certain patterns. The ratio (Al+Si):O is 1:2. In calcic zeolites, the ratio (Ca:Al) is always 1:2, and in alkali zeolites, the ratio (Na,K):Al is always 1:1. Zeolites and feldspathoids are closely related, but zeolites have more open structures and contain loosely bonded H2O compared with feldspathoids. Sodalite is sometimes grouped with the feldspathoids rather than the zeolites, but it has a zeolite-type structure and contains loosely bonded NaCl. Analcime, NaAlSi2O6∙H2O, is also sometimes considered a zeolite, but is closer to leucite and other feldspathoids in atomic structure.
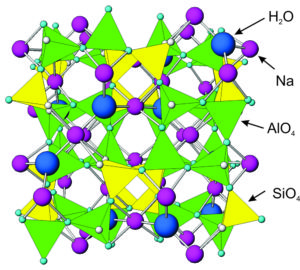
Figure 7.57 shows the atomic arrangement in analcime – it is similar in most ways to all zeolites. All zeolites contain tetrahedra with aluminum and silicon cations surrounded by four oxygen anions. Corners of the tetrahedra join to other corners.
Analcime and other zeolites contain holes and channels between tetrahedra that can hold or transmit large ions, such as sodium, calcium, potassium, H2O, or other molecules. This property sets them apart from most other minerals. Different zeolites have different-sized openings, and in some zeolites the openings connect to form channels. Because of the cavities and channels, zeolites have many industrial applications. See Box 7-4 (below).
7.4.7 Chert
Chert is a fine-grained (microcrystalline) variety of quartz. It is also the name given to rock composed primarily of fine-grained quartz. So, the name is used in two ways. Chert (the silica mineral kind) may be massive or layered. It is often in nodules or concretions in limestone. Some chert forms by recrystallization of amorphous silica. Chert has many appearances; the five photos below (Figures 7.59 to 7.63) show examples of some of the more common varieties. Common chert is light grey. Jasper is chert with a characteristic red color due to hematite inclusions. Flint, a darker form of chert, contains organic matter. Opal and chalcedony, two other types of silica, are often associated with chert deposits. From the Stone Age until the Industrial Revolution, chert and flint were highly valued for use as weapons, tools, and fire starters.
7.5 Common Sedimentary Rocks
Sediments and sedimentary rocks cover about 80% of all continental areas but are less than 1% of the volume of the Earth’s crust. They are, in effect, a thin blanket on top of igneous and metamorphic basement rocks. Yet, we interact with them more than with any other geological materials except, perhaps, soils. Sediments, and rocks derived from sediments, are mostly recycled materials that come from preexisting igneous, metamorphic, or sedimentary rocks. Petrologists usually divide sedimentary rocks into two main groups based on the kinds of material they comprise: clastic rocks and chemical rocks.
7.5.1 Clastic Sedimentary Rocks
Clastic sedimentary rocks are formed by compaction and cementation of clasts composed of individual mineral grains or pieces of rock. Because their mineralogy varies so much, we generally classify clastic rocks based on grain size rather than composition. Grain size varies from huge clasts and boulders in gravels and conglomerates, to fine “clay size” (<0.004 mm) particles in muds and shales. The table below gives a standard classification scheme for clastic rocks based on clast/grain size. See section 7.2 (above), for more specifics about grain sizes.
| Classification of Clastic Rocks Based on Clast Size | |||||
| clast size | sedimentary rock | major component of unconsolidated sediment | clast sizes | ||
| very fine | mudrocks | mudstone | claystone | clay | more than 60% clay-sized |
| siltstone | silt, generally quartz | more than 60% clay-sized | |||
| shale | clay | clay- and silt-sized | |||
| small to medium | sandstone | sand, commonly quartz | sand | ||
| very coarse | conglomerate or breccia |
gravel or coarser sediment | pebble | ||
| gravel or coarser sediment | cobble | ||||
| gravel or coarser sediment | boulder | ||||
In the coarsest clastic rocks, a fine-grained matrix separates large clasts that may be of many different compositions. If the clasts are angular, the rock is a breccia; if they are rounded, it is a conglomerate. Lithic fragments generally dominate the clasts in conglomerate and breccia. Figures 7.65 and 7.66 show a conglomerate and a breccia; both are from Death Valley National Park.
Rocks containing small- to medium-sized grains are generally called sandstone. The photos below in Figures 7.67, 7.68, and 7.69 show three examples. Most sandstones contain sand-sized (0.062 to 2 mm in longest dimension) quartz or feldspar grains. Coarser sandstones may contain both lithic fragments and individual detrital mineral grains. Sometimes clays or other minerals are present in a matrix between larger grains. We call a sandstone that is entirely, or almost entirely made of quartz, an arenite. The rock in Figure 7.67 is an example. We call sandstones containing significant amounts of matrix material or lithic fragments, such as the rock seen in Figure 7.68, wackes, or graywackes. Arkoses, like the one seen in Figure 7.69, are sandstones that contain significant amounts of K-feldspar.
Conglomerates and sandstones together account for 20% to 25% of all sedimentary rocks. Finer-grained clastic rocks, generally termed mudrocks, are much more common. These fine-grained rocks consist of microscopic (<0.062 mm in longest dimension) clay and quartz grains. Lithic fragments are absent.

We call the finest grained rocks shale if they exhibit fissility. Fissility, the ability to cleave into very thin layers, results from parallel alignment of clay grains. This photo (Figure 7.70) shows thin pieces of the Marcellus Shale from Pennsylvania.
We call very fine-grained rocks that are not fissile mudstones. We further divide mudstones into claystones or siltstones depending if they are clay-rich or quartz-rich, respectively.
| Minerals in Mudrocks and Sandstones |
||
| Minerals | Mudrocks average % |
Sandstones average % |
| clay minerals | 60 | 5 |
| quartz | 30 | 65 |
| feldspar | 4 | 10-15 |
| carbonate minerals | 3 | <1 |
Since clastic sediments can be derived from any preexisting rocks, they may contain a variety of minerals and rock fragments. However, only a few minerals are common. The table compares the most common minerals in mudrocks and sandstones. Clays, with subordinate quartz, dominate the mudrocks, but quartz dominates the sandstones.
Quartz, feldspar, and lithic fragments containing quartz and feldspar comprise all but the finest grained rocks because they are resistant to weathering. We call rocks dominated by quartz quartzose, while we term those containing large amounts of feldspar feldspathic, or (if they are rich in K-feldspar) arkosic. In mudstones and shales, clay minerals dominate. In all these rocks, other minerals, including micas, magnetite, rutile, ilmenite, sphene, zircon, apatite, or garnet may occasionally be significant components. Carbonate grains and organic material may be present as well. Because most minerals break down to clay and quartz if exposed at the Earth surface for sufficient time, it should be no surprise that mudstone and shale, which are composed primarily of clay and quartz, are the most abundant sedimentary rocks.

In the coarser-grained clastic rocks, the compositions of lithic fragments give clues to the origin of the sediment. In the finer-grained rocks, mineralogical composition is often difficult to determine and interpret. Suppose we look at a sandstone with a hand lens (Figure 7.71). Quartz, and perhaps K-feldspar, may be easy to find. However, lithic fragments can be confusing and small grains of chert or magnetite may be impossible to identify. For fine sandstones and finer-grained rocks, mineral identification can be problematic, even with a petrographic microscope. Distinguishing quartz from feldspar and telling clay minerals apart may be impossible without using an X-ray diffractometer or other sophisticated equipment.
7.5.2 Chemical Sedimentary Rocks
Chemical sedimentary rocks are formed by precipitation of minerals from water or by alteration of already existing material in place. The most common of these rocks include evaporites, chert, and some varieties of carbonate rocks (limestones and dolostones). In contrast with clastic sedimentary rocks, petrologists name chemical sedimentary rocks based on chemical composition. This is because chemical sedimentary rocks usually include only one or a few minerals – the chemical processes that form them tend to isolate certain elements. The most common precipitated minerals consist of elements of high solubility (for example, alkalis such as sodium or potassium) or elements of great abundance (for example, silicon). Below we look at some of the different kinds of chemical sedimentary rocks in more detail.
7.5.2.1 Carbonate Rocks


While limestone is a general term given to all carbonate rocks, we use the names dolomite or dolostone for rocks in which dolomite is the dominant carbonate mineral. Limestone and dolostone account for 10% to 15% of all sedimentary rocks. They are mostly composed of calcite or dolomite, one or the other. Surprisingly, rocks with significant amounts of both calcite and dolomite are rare. Besides calcite and dolomite, carbonate rocks often contain detrital quartz or clay minerals and sometimes authigenic minerals of many kinds. Both limestones and dolostones easily weather or dissolve, producing distinctive textures such as seen in Figures 7.72 and 7.73. Commonly cracks appear enlarged, and edges are rounded.
Distinguishing limestone from dolostone, based on appearance, can be difficult or impossible in outcrop. On a fresh surface calcite and dolomite may look the same, although weathering sometimes alters dolomite to a yellow-brown color that is distinctive. Field geologists carry dilute hydrochloric acid – which reacts only with calcite or powdered dolomite – or chemical stains to help identification of carbonate minerals. Aragonite, a polymorph of calcite, exists in some very young carbonate deposits, but never in old rocks because it changes to calcite over time. In contrast, for reasons that are not completely clear, dolomite is rare in modern carbonates but is common in Paleozoic and Precambrian rocks.

When CaCO3 precipitates to form limestones, it may be as fine-grained lime muds, resulting in microcrystalline calcite, called micrite. It also precipitates as coarser sparry calcite, with crystals that may be clear and easily visible with the naked eye. However, most carbonate rocks are detrital. They form from organic debris deposited in shallow marine environments, where most biological activity occurs. These rocks often contain fossils amidst clastic grains. The fossils can be of many different sorts. The limestone in Figure 7.74 contains conspicuous ribbed brachiopods.
7.5.2.2 Evaporites
Earlier in this chapter, we discussed evaporite minerals that may accumulate in deposits that are meters or many meters thick. The most common of these deposits contain beds of halite or gypsum. Rocks composed of these two minerals are found in many places. Halite deposits may be 1,000 m thick or more, but gypsum rock deposits are generally much thinner. Both kinds of deposits form during evaporation of inland seas or other isolated waters. For example, halite and gypsum are mined in Michigan and Ontario where the minerals collected when water trapped in an inland basin, called the Michigan Basin, evaporated 360-440 million years ago. The world’s largest underground salt mine is in Goderich, Ontario, on the edge of the basin.
7.5.2.3 Chert

We talked about varieties of mineralogical chert in Section 7.4.7 above. Chert is also the name given to hard sedimentary rock composed of fine quartz crystals. The rock seen in Figure 7.75 is an example. Chert (the rock) is usually of biological origin, being the petrified remains of siliceous ooze, the biogenic sediment that covers large areas of the deep ocean floor. Chert often contains microscopic skeletal remains of diatoms, silicoflagellates, and radiolarians made of silica. Depending on its origin, chert can contain either microfossils, small macrofossils, or both. Chert (the rock) varies greatly in color (from white to black), but most often manifests as gray, brown, grayish brown and light green to rusty red (occasionally dark green too). Its color is an expression of trace elements present in the rock, and both red and green colors are most often related to small amounts of iron (in its oxidized and reduced forms respectively). Thick beds of chert, up to several hundred meters thick, underlie most of the southern plains states of the United States and parts of California. For poorly understood reasons, thick chert beds are especially common in Precambrian rocks.
7.5.2.4 Other Chemical Sedimentary Rocks
Many other types of chemical sedimentary rocks and minerals exist. They are not abundant, but they may be important as sources of ore. Collophane, a cryptocrystalline form of apatite, Ca5(PO4)3(OH,F,Cl), is found in small amounts in many kinds of sedimentary rocks. It has an organic origin, and sometimes it becomes concentrated. In rocks called phosphorites, collophane/apatite may comprise nearly the entire rock. Phosphorite mines in Wyoming and Idaho produce phosphate, an important component of fertilizers.

Iron formations, most Precambrian in age, are mined for iron in the Mesabi Range of Minnesota and elsewhere. These rocks are mostly formed from chemical sediments. Iron oxides and hydroxides, including hematite (Fe2O3), goethite (FeO(OH)) and magnetite (Fe3O4), or Fe-carbonate (siderite, FeCO3), are the most common iron minerals in iron formations. Chert is also a common component. Occasionally, iron silicates (e.g., fayalite, Fe2SiO4) or sulfide (pyrite, FeS2) may be present. The outcrop seen in Figure 7.76 is iron formation that contains layers rich in red chert alternating with layers rich in specular hematite (silver grey). Iron formations form from sediments originally deposited in shallow marine conditions. Young manganese deposits, similar to iron formations, have been dredged from ocean floors.
7.5.3 Organic Sedimentary Rocks

Some sedimentary rocks are formed largely from biogenic (organic) debris. We classify such rocks as organic sedimentary rocks, separate from chemical and clastic sedimentary rocks. Examples are limestones formed from shell or skeletal remains, coquina (a sedimentary rock made of shell fragments), diatomite (a sedimentary rock made of the remains of diatoms), and coal. This photo (Figure 7.77) shows an example of coquina.
Much overlap exists between chemical, clastic, and organic sedimentary rocks. Many chemical sedimentary rocks contain clastic material, and many clastic sedimentary rocks are held together by chemical cements precipitated from water. Both chemical and clastic rocks may contain biogenic components.
7.6 Sedimentary Environments and Facies
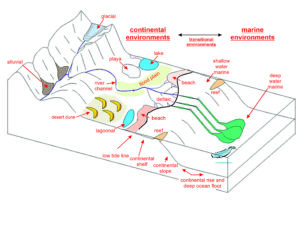
The processes that form sedimentary rock occur in many environments, and different minerals or rocks can characterize each. In many places, however, the rate of sediment deposition is slow. In other places, erosion removes sediment as fast as it is deposited. Consequently, most of the sedimentary rocks and minerals that we see are associated with a few special environments where the rate of deposition is relatively fast, and the volume of sediments deposited was relatively large. The most significant such environments are shallow seas and large basins on continents. Lesser amounts of sedimentary rocks are associated with other environments such as shorelines, rivers, lakes, deserts, and glaciers. Because different environments are different physically, chemically, and biologically, the nature of sedimentary rocks is highly variable. The most significant factors that account for the differences are the energy and biology of the environment, the distance from the source of the sediment, and the way the sediment was transported. Figure 7.78 show some of the many different places where sediments are deposited. Sedimentologists divide all of them into smaller categories that produce distinctive sediments and sedimentary rocks.
Most sedimentary environments vary laterally. Large basins, for example, are not the same everywhere. So different kinds of sediment can be deposited in different parts of a basin simultaneously. We use the term facies to refer to the different rocks that characterize a particular process or environment. So, geologists may talk about continental, transitional, or marine facies. More specifically, they may consider reef facies, continental shelf facies, deep ocean facies, and so on.
Different contemporaneous facies often grade into each other. Consider, for example, sedimentation at or near a shoreline. Coarse sediments are typically deposited on dry land and on beaches, somewhat finer sediments in shallow water, and the finest materials farther offshore. If the sedimentary environment changes, the nature of sedimentation changes. With rising sea level, for example, fine deep-water sediments may be deposited over coarser shoreline sediments. With failing sea level, the opposite may occur. So, sedimentary facies vary both laterally and vertically (geographically and temporally).
blank line
blank line
●Figure CreditsUncredited graphics/photos came from the authors and other primary contributors to this book. 7.1 Salt deposits on the shore of the Dead Sea, Jordan, Gerda Arendt, Wikimedia Commons |