Announcement: Chapter quizzes are not working as of summer 2023.

KEY CONCEPTS
At the end of this chapter, students should be able to:
- Define mineral.
- Describe the basic structure of the atom.
- Derive basic atomic information from the Periodic Table of Elements.
- Describe chemical bonding related to minerals.
- Describe the main ways minerals form.
- Describe the silicon-oxygen tetrahedron and how it forms common silicate minerals.
- List common non-silicate minerals in oxide, sulfide, sulfate, and carbonate groups.
- Identify minerals using physical properties and identification tables.
The term “minerals” as used in nutrition labels and pharmaceutical products is not the same as a mineral in a geological sense. In geology, the classic definition of a mineral is: 1) naturally occurring, 2) inorganic, 3) solid at room temperature, 4) regular crystal structure, and 5) defined chemical composition. Some natural substances technically should not be considered minerals, but are included by exception. For example, water and mercury are liquid at room temperature. Both are considered minerals because they were classified before the room-temperature rule was accepted as part of the definition. Calcite is quite often formed by organic processes, but is considered a mineral because it is widely found and geologically important. Because of these discrepancies, the International Mineralogical Association in 1985 amended the definition to: “A mineral is an element or chemical compound that is normally crystalline and that has been formed as a result of geological processes.” This means that the calcite in the shell of a clam is not considered a mineral. But once that clam shell undergoes burial, diagenesis, or other geological processes, then the calcite is considered a mineral. Typically, substances like coal, pearl, opal, or obsidian that do not fit the definition of mineral are called mineraloids.
A rock is a substance that contains one or more minerals or mineraloids. As is discussed in later chapters, there are three types of rocks composed of minerals: igneous (rocks crystallizing from molten material), sedimentary (rocks composed of products of mechanical weathering (sand, gravel, etc.) and chemical weathering (things precipitated from solution), and metamorphic (rocks produced by alteration of other rocks by heat and pressure.
3.1 Chemistry of Minerals
Rocks are composed of minerals that have a specific chemical composition. To understand mineral chemistry, it is essential to examine the fundamental unit of all matter, the atom.
3.1.1 The Atom
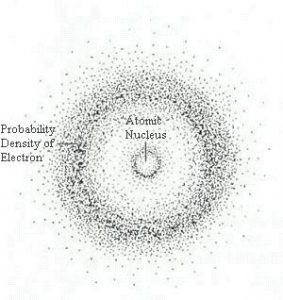
Matter is made of atoms. Atoms consists of subatomic particles—protons, neutrons, and electrons. A simple model of the atom has a central nucleus composed of protons, which have positive charges, and neutrons which have no charge. A cloud of negatively charged electrons surrounds the nucleus, the number of electrons equaling the number of protons thus balancing the positive charge of the protons for a neutral atom. Protons and neutrons each have a mass number of 1. The mass of an electron is less than 1/1000th that of a proton or neutron, meaning most of the atom’s mass is in the nucleus.
3.1.2 Periodic Table of the Elements
Matter is composed of elements which are atoms that have a specific number of protons in the nucleus. This number of protons is called the Atomic Number for the element. For example, an oxygen atom has 8 protons and an iron atom has 26 protons. An element cannot be broken down chemically into a simpler form and retains unique chemical and physical properties. Each element behaves in a unique manner in nature. This uniqueness led scientists to develop a periodic table of the elements, a tabular arrangement of all known elements listed in order of their atomic number.
![By OpenStax Anatomy and PhysiologyOpenStax [<a href="http://creativecommons.org/licenses/by/4.0">CC BY 4.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3A203_Periodic_Table-02.jpg">via Wikimedia Commons</a> The Periodic Table of the Elements showing all elements with their chemical symbols, atomic weight, and atomic number.](https://opengeology.org/textbook/wp-content/uploads/2016/07/Periodic_Table-02-1024x795.jpg)
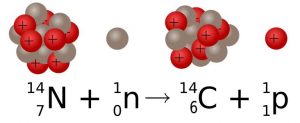
The atomic mass of natural elements represents an average mass of the atoms comprising that substance in nature and is usually not a whole number as seen on the periodic table, meaning that an element exists in nature with atoms having different numbers of neutrons. The differing number of neutrons affects the mass of an element in nature and the atomic mass number represents this average. This gives rise to the concept of isotope. Isotopes are forms of an element with the same number of protons but different numbers of neutrons. There are usually several isotopes for a particular element. For example, 98.9% of carbon atoms have 6 protons and 6 neutrons. This isotope of carbon is called carbon-12 (12C). A few carbon atoms, carbon-13 (13C), have 6 protons and 7 neutrons. A trace amount of carbon atoms, carbon-14 (14C), has 6 protons and 8 neutrons.
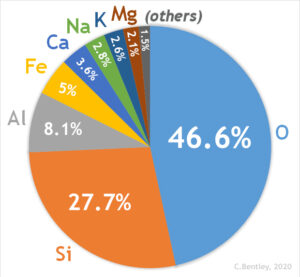
Among the 118 known elements, the heaviest are fleeting human creations known only in high energy particle accelerators, and they decay rapidly. The heaviest naturally occurring element is uranium, atomic number 92. The eight most abundant elements in Earth’s continental crust are shown in Table 1. These elements are found in the most common rock forming minerals.
| Element | Symbol | Abundance % |
| Oxygen | O | 47% |
| Silicon | Si | 28% |
| Aluminum | Al | 8% |
| Iron | Fe | 5% |
| Calcium | Ca | 4% |
| Sodium | Na | 3% |
| Potassium | K | 3% |
| Magnesium | Mg | 2% |
Table 1. Eight Most Abundant Elements in the Earth’s Continental Crust % by weight (source: USGS). All other elements are less than 1%.
3.1.3 Chemical Bonding
![Source: By Dan Craggs (Own work) [<a href="http://creativecommons.org/publicdomain/zero/1.0/deed.en">CC0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AH2O_2D_labelled.svg">via Wikimedia Commons</a> The hydrogen atoms are on one side, about 105° apart.](https://opengeology.org/textbook/wp-content/uploads/2017/02/H2O_2D_labelled.svg_-300x131.png)
Most minerals are also compounds of more than one element. The common mineral calcite has the chemical formula CaCO3 indicating the molecule consists of one calcium, one carbon, and three oxygen atoms. In calcite, one carbon and three oxygen atoms are held together by covalent bonds to form a molecular ion, called carbonate, which has a negative charge. Calcium as an ion has a positive charge of plus two. The two oppositely charged ions attract each other and combine to form the mineral calcite, CaCO3. The name of the chemical compound is calcium carbonate, where calcium is Ca and carbonate refers to the molecular ion CO3-2.
The mineral olivine has the chemical formula (Mg,Fe)2SiO4, in which one silicon and four oxygen atoms are bonded with two atoms of either magnesium or iron. The comma between iron (Fe) and magnesium (Mg) indicates the two elements can occupy the same location in the crystal structure and substitute for one another.
3.1.3.1 Valence and Charge
The electrons around the atom’s nucleus are located in shells representing different energy levels. The outermost shell is called the valence shell. Electrons in the valence shell are involved in chemical bonding. In 1913, Niels Bohr proposed a simple model of the atom that states atoms are more stable when their outermost shell is full. Atoms of most elements thus tend to gain or lose electrons so the outermost or valence shell is full. In Bohr’s model, the innermost shell can have a maximum of two electrons and the second and third shells can have a maximum of eight electrons. When the innermost shell is the valence shell, as in the case of hydrogen and helium, it obeys the octet rule when it is full with two electrons. For elements in higher rows, the octet rule of eight electrons in the valence shell applies.
![Source: By Jynto [<a href="http://creativecommons.org/publicdomain/zero/1.0/deed.en">CC0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ACarbon_dioxide_3D_ball.png">via Wikimedia Commons</a> Carbon dioxide molecule with a carbon ion in the center and two oxygen ions on either side, each sharing two electrons with the carbon.](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.4_Carbon_dioxide_3D_ball-300x213.png)
In row 3 and column I, sodium (Na) has 11 protons in the nucleus and 11 electrons in three shells—2 electrons in the inner shell, 8 electrons in the second shell, and 1 electron in the valence shell. To maintain a full outer shell of 8 electrons per the octet rule, sodium readily gives up that 1 electron so there are 10 total electrons. With 11 positively charged protons in the nucleus and 10 negatively charged electrons in two shells, sodium when forming chemical bonds is an ion with an overall net charge of +1.
All elements in column I have a single electron in their valence shell and a valence of 1. These other column I elements also readily give up this single valence electron and thus become ions with a +1 charge. Elements in column II readily give up 2 electrons and end up as ions with a charge of +2. Note that elements in columns I and II which readily give up their valence electrons, often form bonds with elements in columns VI and VII which readily take up these electrons. Elements in columns 3 through 15 are usually involved in covalent bonding. The last column 18 (VIII) contains the noble gases. These elements are chemically inert because the valence shell is already full with 8 electrons, so they do not gain or lose electrons. An example is the noble gas helium which has 2 valence electrons in the first shell. Its valence shell is therefore full. All elements in column VIII possess full valence shells and do not form bonds with other elements.
As seen above, an atom with a net positive or negative charge as a result of gaining or losing electrons is called an ion. In general the elements on the left side of the table lose electrons and become positive ions, called cations because they are attracted to the cathode in an electrical device. The elements on the right side tend to gain electrons. These are called anions because they are attracted to the anode in an electrical device. The elements in the center of the periodic table, columns 3 through 15, do not consistently follow the octet rule. These are called transition elements. A common example is iron, which has a +2 or +3 charge depending on the oxidation state of the element. Oxidized Fe+3 carries a +3 charge and reduced Fe+2 is +2. These two different oxidation states of iron often impart dramatic colors to rocks containing their minerals—the oxidized form producing red colors and the reduced form producing green.
3.1.3.2 Ionic Bonding
![Source: By Benjah-bmm27 (Own work) [Public domain], <a href="https://commons.wikimedia.org/wiki/File%3ASodium-chloride-3D-ionic.png">via Wikimedia Commons</a> Image of crystal model of halite with ions of sodium and chlorine arranged in a cubic structure.](https://opengeology.org/textbook/wp-content/uploads/2016/07/03-Sodium-chloride-3D-ionic-300x284.png)
3.1.3.3 Covalent Bonding
![By DynaBlast (Created with Inkscape) [<a href="http://creativecommons.org/licenses/by-sa/2.5">CC BY-SA 2.5</a>], <a href="https://commons.wikimedia.org/wiki/File%3ACovalent.svg">via Wikimedia Commons</a> Each atom is sharing electrons.](https://opengeology.org/textbook/wp-content/uploads/2016/07/Covalent.svg_-249x300.png)
[ays_quiz id=”17″]
3.2 Formation of Minerals
Minerals form when atoms bond together in a crystalline arrangement. Three main ways this occurs in nature are: 1) precipitation directly from an aqueous (water) solution with a temperature change, 2) crystallization from a magma with a temperature change, and 3) biological precipitation by the action of organisms.
3.2.1 Precipitation from aqueous solution
![Source: By Bbypnda (Own work) [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AHard_Water_Calcification.jpg">via Wikimedia Commons</a> Encrusted calcium carbonate (lime) deposits on faucent](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.5_Hard_Water_Calcification-300x200.jpg)
Precipitation is the reverse process, in which ions in solution come together to form solid minerals. Precipitation is dependent on the concentration of ions in solution and other factors such as temperature and pressure. The point at which a solvent cannot hold any more solute is called saturation. Precipitation can occur when the temperature of the solution falls, when the solute evaporates, or with changing chemical conditions in the solution. An example of precipitation in our homes is when water evaporates and leaves behind a rind of minerals on faucets, shower heads, and drinking glasses.
In nature, changes in environmental conditions may cause the minerals dissolved in water to form bonds and grow into crystals or cement grains of sediment together. In Utah, deposits of tufa formed from mineral-rich springs that emerged into the ice age Lake Bonneville. Now exposed in dry valleys, this porous tufa was a natural insulation used by pioneers to build their homes with a natural protection against summer heat and winter cold. The travertine terraces at Mammoth Hot Springs in Yellowstone Park are another example formed by calcite precipitation at the edges of the shallow spring-fed ponds.

Another example of precipitation occurs in the Great Salt Lake, Utah, where the concentration of sodium chloride and other salts is nearly eight times greater than in the world’s oceans [zotpressInText item=”{DU5CMSHJ}” format=”%num%” brackets=”yes”]. Streams carry salt ions into the lake from the surrounding mountains. With no other outlet, the water in the lake evaporates and the concentration of salt increases until saturation is reached and the minerals precipitate out as sediments. Similar salt deposits include halite and other precipitates, and occur in other lakes like Mono Lake in California and the Dead Sea.
3.2.2 Crystallization from Magma
![Source: By Hawaii Volcano Observatory (DAS) [Public domain], <a href="https://commons.wikimedia.org/wiki/File%3APahoehoe_toe.jpg">via Wikimedia Commons</a> A lava flow](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.5a_Pahoehoe_toe-300x188.jpg)
3.2.3 Precipitation by Organisms
![Source: By ermell (Own work) [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AAmmonit-9133155-PS.jpg">via Wikimedia Commons</a> Shell of an ammonite, an extinct cephalopod, with a spiral shell in a plane.](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.8_Ammonite_Asteroceras-300x225.jpg)
[ays_quiz id=”18″]
3.3 Silicate Minerals
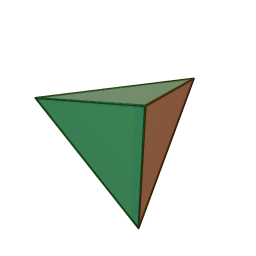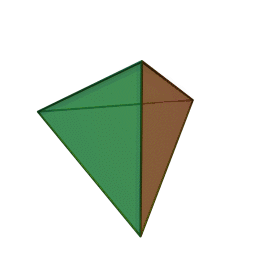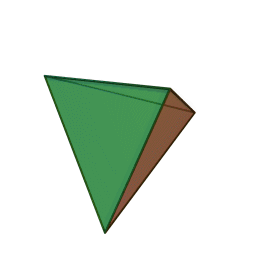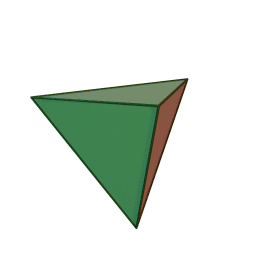
Minerals are categorized based on their composition and structure. Silicate minerals are built around a molecular ion called the silicon-oxygen tetrahedron. A tetrahedron has a pyramid-like shape with four sides and four corners. Silicate minerals form the largest group of minerals on Earth, comprising the vast majority of the Earth’s mantle and crust. Of the nearly four thousand known minerals on Earth, most are rare. There are only a few that make up most of the rocks likely to be encountered by surface dwelling creatures like us. These are generally called the rock-forming minerals.

The silicon-oxygen tetrahedron (SiO4) consists of a single silicon atom at the center and four oxygen atoms located at the four corners of the tetrahedron. Each oxygen ion has a -2 charge and the silicon ion has a +4 charge. The silicon ion shares one of its four valence electrons with each of the four oxygen ions in a covalent bond to create a symmetrical geometric four-sided pyramid figure. Only half of the oxygen’s valence electrons are shared, giving the silicon-oxygen tetrahedron an ionic charge of -4. This silicon-oxygen tetrahedron forms bonds with many other combinations of ions to form the large group of silicate minerals.

The silicon ion is much smaller than the oxygen ions (see the figures) and fits into a small space in the center of the four large oxygen ions, seen if the top ball is removed (as shown in the figure to the right). Because only one of the valence electrons of the corner oxygens is shared, the silicon-oxygen tetrahedron has chemically active corners available to form bonds with other silica tetrahedra or other positively charged ions such as Al+3, Fe+2,+3, Mg+2, K+1, Na+1, and Ca+2. Depending on many factors, such as the original magma chemistry, silica-oxygen tetrahedra can combine with other tetrahedra in several different configurations. For example, tetrahedra can be isolated, attached in chains, sheets, or three dimensional structures. These combinations and others create the chemical structure in which positively charged ions can be inserted for unique chemical compositions forming silicate mineral groups.
3.3.1 The dark ferromagnesian silicates
![Source: Vsmith assumed (based on copyright claims). [<a href="http://www.gnu.org/copyleft/fdl.html">GFDL</a>, <a href="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0</a> or <a href="http://creativecommons.org/licenses/by-sa/2.5">CC BY-SA 2.5</a>], <a href="https://commons.wikimedia.org/wiki/File%3APeridot_in_basalt.jpg">via Wikimedia Commons</a> Many small crystall of the green mineral olivine in a mass of basalt](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.12_Peridot_in_basalt-300x225.jpg)
Olivine is the primary mineral component in mantle rock such as peridotite and basalt. It is characteristically green when not weathered. The chemical formula is (Fe,Mg)2SiO4. As previously described, the comma between iron (Fe) and magnesium (Mg) indicates these two elements occur in a solid solution. Not to be confused with a liquid solution, a solid solution occurs when two or more elements have similar properties and can freely substitute for each other in the same location in the crystal structure.
Olivine is referred to as a mineral family because of the ability of iron and magnesium to substitute for each other. Iron and magnesium in the olivine family indicates a solid solution forming a compositional series within the mineral group which can form crystals of all iron as one end member and all mixtures of iron and magnesium in between to all magnesium at the other end member. Different mineral names are applied to compositions between these end members. In the olivine series of minerals, the iron and magnesium ions in the solid solution are about the same size and charge, so either atom can fit into the same location in the growing crystals. Within the cooling magma, the mineral crystals continue to grow until they solidify into igneous rock. The relative amounts of iron and magnesium in the parent magma determine which minerals in the series form. Other rarer elements with similar properties to iron or magnesium, like manganese (Mn), can substitute into the olivine crystalline structure in small amounts. Such ionic substitutions in mineral crystals give rise to the great variety of minerals and are often responsible for differences in color and other properties within a group or family of minerals. Olivine has a pure iron end-member (called fayalite) and a pure magnesium end-member (called forsterite). Chemically, olivine is mostly silicon, oxygen, iron, and magnesium and therefore is grouped among the dark-colored ferromagnesian (iron=ferro, magnesium=magnesian) or mafic minerals, a contraction of their chemical symbols Ma and Fe. Mafic minerals are also referred to as dark-colored ferromagnesian minerals. Ferro means iron and magnesian refers to magnesium. Ferromagnesian silicates tend to be more dense than non-ferromagnesian silicates. This difference in density ends up being important in controlling the behavior of the igneous rocks that are built from these minerals: whether a tectonic plate subducts or not is largely governed by the density of its rocks, which are in turn controlled by the density of the minerals that comprise them.
The crystal structure of olivine is built from independent silica tetrahedra. Minerals with independent tetrahedral structures are called neosilicates (or orthosilicates). In addition to olivine, other common neosilicate minerals include garnet, topaz, kyanite, and zircon.
Two other similar arrangements of tetrahedra are close in structure to the neosilicates and grade toward the next group of minerals, the pyroxenes. In a variation on independent tetrahedra called sorosilicates, there are minerals that share one oxygen between two tetrahedra, and include minerals like pistachio-green epidote, a gemstone. Another variation are the cyclosilicates, which as the name suggests, consist of tetrahedral rings, and include gemstones such as beryl, emerald, aquamarine, and tourmaline
3.3.2 Pyroxene Family
![Source: Rob Lavinsky, <a rel="nofollow" class="external text" href="http://www.irocks.com/">iRocks.com</a> – CC-BY-SA-3.0 [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ADiopside-172005.jpg">via Wikimedia Commons</a> Dark green crystals of diopside, a member of the pyroxene family](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.14_Diopside-172005-300x231.jpg)
![Source: By Bubenik (Own work) [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3APyroxen-chain.png">via Wikimedia Commons</a> Single chain of tetrahedra in pyroxene, alternating with adjacent corner oxygens bonded. The outer corners are active to bond with other anions.](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.15_Pyroxen-chain-41x300.png)
Pyroxenes are built from long, single chains of polymerized silica tetrahedra in which tetrahedra share two corner oxygens. The silica chains are bonded together into the crystal structures by metal cations. A common member of the pyroxene family is augite, itself containing several solid solution series with a complex chemical formula (Ca,Na)(Mg,Fe,Al,Ti)(Si,Al)2O6 that gives rise to a number of individual mineral names.
This single-chain crystalline structure bonds with many elements, which can also freely substitute for each other. The generalized chemical composition for pyroxene is XZ(Al,Si)2O6. X represents the ions Na, Ca, Mg, or Fe, and Z represents Mg, Fe, or Al. These ions have similar ionic sizes, which allows many possible substitutions among them. Although the cations may freely substitute for each other in the crystal, they carry different ionic charges that must be balanced out in the final crystalline structure. For example Na has a charge of +1, but Ca has charge of +2. If a Na+ ion substitutes for a Ca+2 ion, it creates an unequal charge that must be balanced by other ionic substitutions elsewhere in the crystal. Note that ionic size is more important than ionic charge for substitutions to occur in solid solution series in crystals.
3.3.3 Amphibole Family

![Solurce: By Dave Dyet http://www.shutterstone.com http://www.dyet.com (Own work) [Public domain], <a href="https://commons.wikimedia.org/wiki/File%3AOrthoclase_Hornblende.jpg">via Wikimedia Commons</a> A crystal of orthoclase (potassium feldspar) wth elongated dark crystals of hornblende](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.15_Orthoclase_Hornblende-300x300.jpg)
![Source: By Bubenik (Own work) [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ATremolite-chain.png">via Wikimedia Commons</a> Double chain structure of amphibole; two single chains laying together with the inner corners of each tetrahedron bonded and the outer cornera active to bond with anions](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.17_Tremolite-chain-79x300.png)
3.3.4 Sheet Silicates


Sheet silicates are built from tetrahedra which share all three of their bottom corner oxygens thus forming sheets of tetrahedra with their top corners available for bonding with other atoms. Micas and clays are common types of sheet silicates, also known as phyllosilicates. Mica minerals are usually found in igneous and metamorphic rocks, while clay minerals are more often found in sedimentary rocks. Two frequently found micas are dark-colored biotite, frequently found in granite, and light-colored muscovite, found in the metamorphic rock called schist.
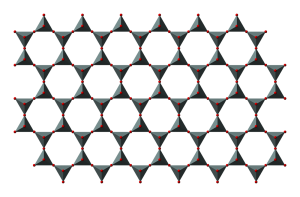
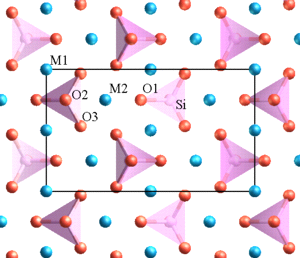
Chemically, sheet silicates usually contain silicon and oxygen in a 2:5 ratio (Si4O10). Micas contain mostly silica, aluminum, and potassium. Biotite mica has more iron and magnesium and is considered a ferromagnesian silicate mineral. Muscovite micas belong to the felsic silicate minerals. Felsic is a contraction formed from feldspar, the dominant mineral in felsic rocks.
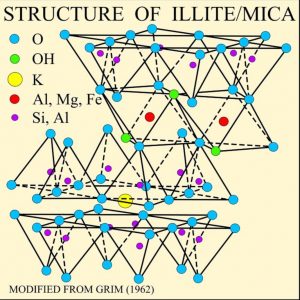
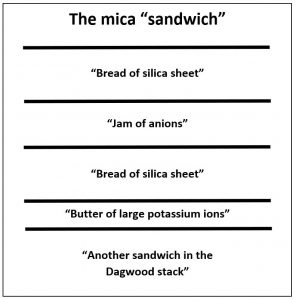
The illustration of the crystalline structure of mica shows the corner O atoms bonded with K, Al, Mg, Fe, and Si atoms, forming polymerized sheets of linked tetrahedra, with an octahedral layer of Fe, Mg, or Al, between them. The yellow potassium ions form Van der Waals bonds (attraction and repulsion between atoms, molecules, and surfaces) and hold the sheets together. Van der Waals bonds differ from covalent and ionic bonds, and exist here between the sandwiches, holding them together into a stack of sandwiches. The Van der Waals bonds are weak compared to the bonds within the sheets, allowing the sandwiches to be separated along the potassium layers. This gives mica its characteristic property of easily cleaving into sheets.
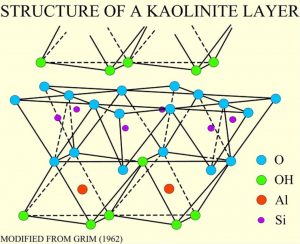
Clays minerals occur in sediments formed by the weathering of rocks and are another family of silicate minerals with a tetrahedral sheet structure. Clay minerals form a complex family, and are an important component of many sedimentary rocks. Other sheet silicates include serpentine and chlorite, found in metamorphic rocks.
Clay minerals are composed of hydrous aluminum silicates. One type of clay, kaolinite, has a structure like an open-faced sandwich, with the bread being a single layer of silicon-oxygen tetrahedra and a layer of aluminum as the spread in an octahedral configuration with the top oxygens of the sheets.
3.3.5 Framework Silicates
![Source: By Didier Descouens (Own work) [<a href="http://creativecommons.org/licenses/by-sa/4.0">CC BY-SA 4.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AQuartz_Br%C3%A9sil.jpg">via Wikimedia Commons</a> A mass of quartz crystals showing typical six sided habit with points](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.23_-Quartz_Br%C3%A9sil-300x255.jpg)
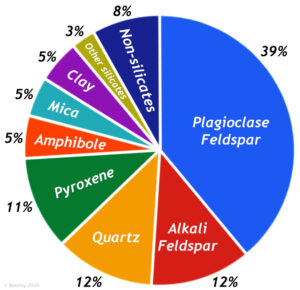
Feldspars are usually found in igneous rocks, such as granite, rhyolite, and basalt as well as metamorphic rocks and detrital sedimentary rocks. Detrital sedimentary rocks are composed of mechanically weathered rock particles, like sand and gravel. Quartz is especially abundant in detrital sedimentary rocks because it is very resistant to disintegration by weathering. While quartz is the most abundant mineral on the Earth’s surface, due to its durability, the feldspar minerals are the most abundant minerals in the Earth’s crust, comprising roughly 50% of the total minerals that make up the crust.
![Source: By Didier Descouens (Own work) [<a href="http://creativecommons.org/licenses/by-sa/4.0">CC BY-SA 4.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AOrthoclaseBresil.jpg">via Wikimedia Commons</a> A group of crystals of pink potassium feldspar](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.24_kspar280x210-1-300x200.jpg)
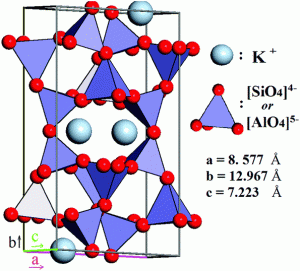
Note that aluminum, which has a similar ionic size to silicon, can substitute for silicon inside the tetrahedra (see figure). Because potassium ions are so much larger than sodium and calcium ions, which are very similar in size, the inability of the crystal lattice to accommodate both potassium and sodium/calcium gives rise to the two families of feldspar, orthoclase and plagioclase respectively. Framework silicates are called tectosilicates and include the alkali metal-rich feldspathoids and zeolites.
[ays_quiz id=”19″]
3.4 Non-Silicate Minerals
![Source: By Matt Affolter(QFL247) (talk) (Transferred by Citypeek/Original uploaded by Matt Affolter(QFL247)) [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a> or <a href="http://www.gnu.org/copyleft/fdl.html">GFDL</a>], <a href="https://commons.wikimedia.org/wiki/File%3AHanksite.JPG">via Wikimedia Commons</a> The mineral is hexagonal and clear.](https://opengeology.org/textbook/wp-content/uploads/2017/01/Hanksite-150x150.jpg)
| Mineral Group | Examples | Formula | Uses |
| Native elements | gold, silver, copper | Au, Ag, Cu | Jewelry, coins, industry |
| Carbonates | calcite, dolomite | CaCO3, CaMg(CO3)2 | Lime, Portland cement |
| Oxides | hematite, magnetite, bauxite | Fe2O3, Fe3O4, a mixture of aluminum oxides | Ores of iron & aluminum, pigments |
| Halides | halite, sylvite | NaCl, KCl | Table salt, fertilizer |
| Sulfides | galena, chalcopyrite, cinnabar | PbS, CuFeS2, HgS | Ores of lead, copper, mercury |
| Sulphates | gypsum, epsom salts | CaSo4·2H2O, MgSO4·7H2O | Sheetrock, therapeutic soak |
| Phosphates | apatite | Ca5(PO4)3(F,Cl,OH) | Fertilizer, teeth, bones |
Common non-silicate mineral groups.
3.4.1 Carbonates


Calcite (CaCO3) and dolomite (CaMg(CO3)2) are the two most frequently occurring carbonate minerals, and usually occur in sedimentary rocks, such as limestone and dolostone rocks, respectively. Some carbonate rocks, such calcite and dolomite, are formed via evaporation and precipitation. However, most carbonate-rich rocks, such as limestone, are created by the lithification of fossilized marine organisms. These organisms, including those we can see and many microscopic organisms, have shells or exoskeletons consisting of calcium carbonate (CaCO3). When these organisms die, their remains accumulate on the floor of the water body in which they live and the soft body parts decompose and dissolve away. The calcium carbonate hard parts become included in the sediments, eventually becoming the sedimentary rock called limestone. While limestone may contain large, easy to see fossils, most limestones contain the remains of microscopic creatures and thus originate from biological processes.
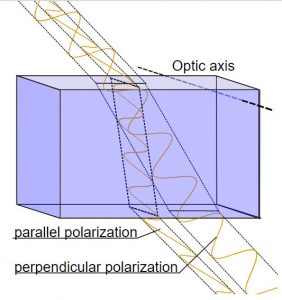
Calcite crystals show an interesting property called birefringence, meaning they polarize light into two wave components vibrating at right angles to each other. As the two light waves pass through the crystal, they travel at different velocities and are separated by refraction into two different travel paths. In other words, the crystal produces a double image of objects viewed through it. Because they polarize light, calcite crystals are used in special petrographic microscopes for studying minerals and rocks.
Many non-silicate minerals are referred to as salts. The term salts used here refers to compounds made by replacing the hydrogen in natural acids. The most abundant natural acid is carbonic acid that forms by the solution of carbon dioxide in water. Carbonate minerals are salts built around the carbonate ion (CO3-2) where calcium and/or magnesium replace the hydrogen in carbonic acid (H2CO3). Calcite and a closely related polymorph aragonite are secreted by organisms to form shells and physical structures like corals. Many such creatures draw both calcium and carbonate from dissolved bicarbonate ions (HCO3–) in ocean water. As seen in the mineral identification section below, calcite is easily dissolved in acid and thus effervesces in dilute hydrochloric acid (HCl). Small dropper bottles of dilute hydrochloric acid are often carried by geologists in the field as well as used in mineral identification labs.
Other salts include halite (NaCl) in which sodium replaces the hydrogen in hydrochloric acid and gypsum (Ca[SO4] • 2 H2O) in which calcium replaces the hydrogen in sulfuric acid. Note that some water molecules are also included in the gypsum crystal. Salts are often formed by evaporation and are called evaporite minerals.

The figure shows the crystal structure of calcite (CaCO3). Like silicon, carbon has four valence electrons. The carbonate unit consists of carbon atoms (tiny white dots) covalently bonded to three oxygen atoms (red), one oxygen sharing two valence electrons with the carbon and the other two sharing one valence electron each with the carbon, thus creating triangular units with a charge of -2. The negatively charged carbonate unit forms an ionic bond with the Ca ion (blue), which as a charge of +2.
3.4.2 Oxides, Halides, and Sulfides

After carbonates, the next most common non-silicate minerals are the oxides, halides, and sulfides.
Oxides consist of metal ions covalently bonded with oxygen. The most familiar oxide is rust, which is a combination of iron oxides (Fe2O3) and hydrated oxides. Hydrated oxides form when iron is exposed to oxygen and water. Iron oxides are important for producing metallic iron. When iron oxide or ore is smelted, it produces carbon dioxide (CO2) and metallic iron.
The red color in rocks is usually due to the presence of iron oxides. For example, the red sandstone cliffs in Zion National Park and throughout Southern Utah consist of white or colorless grains of quartz coated with iron oxide which serve as cementing agents holding the grains together.
![Source: By Dave Dyet http://www.shutterstone.com http://www.dyet.com (Own work) [Public domain], <a href="https://commons.wikimedia.org/wiki/File%3AHematite_-_oolitic_with_shale_Iron_Oxide_Clinton%2C_Oneida_County%2C_New_York.jpg">via Wikimedia Commons</a> A red form of hematite called oolitic showing a mass of small round nodules](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.32_Hematite_-_oolitic_with_shale_Iron_Oxide_Clinton_Oneida_County_New_York-e1512421695503-300x269.jpg)
Other common oxide minerals include:
- ice (H2O), an oxide of hydrogen
- bauxite (Al2H2O4), hydrated oxides of aluminum, an ore for producing metallic aluminum
- corundum (Al2O3), which includes ruby and sapphire gemstones.

The halides consist of halogens in column VII, usually fluorine or chlorine, ionically bonded with sodium or other cations. These include halite or sodium chloride (NaCl), common table salt; sylvite or potassium chloride (KCl); and fluorite or calcium fluoride (CaF2).
![Source: By Famartin (Own work) [<a href="http://creativecommons.org/licenses/by-sa/4.0">CC BY-SA 4.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3A2014-07-05_13_04_30_View_across_the_Bonneville_Salt_Falts%2C_Utah_from_ground_level.JPG">via Wikimedia Commons</a> Photo of salt crust at the Bonneville Salt Flats in Utah with mountains in the background.](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.36_2014-07-05_13_04_30_View_across_the_Bonneville_Salt_Falts_Utah_from_ground_level-300x220.jpg)
Halide minerals usually form from the evaporation of sea water or other isolated bodies of water. A well-known example of halide mineral deposits created by evaporation is the Bonneville Salt Flats, located west of the Great Salt Lake in Utah (see figure).
![Source: By CarlesMillan (Own work) [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3A2780M-pyrite1.jpg">via Wikimedia Commons</a> Cubic crystals of iron pyrite, called "fools gold"](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.37_pyrite1-283x300.jpg)
3.4.3 Sulfates

Sulfate minerals contain a metal ion, such as calcium, bonded to a sulfate ion. The sulfate ion is a combination of sulfur and oxygen (SO4–2). The sulfate mineral gypsum (CaSO4ᐧ2H2O) is used in construction materials such as plaster and drywall. Gypsum is often formed from evaporating water and usually contains water molecules in its crystalline structure. The ᐧ2H2O in the formula indicates the water molecules are whole H2O. This is different from minerals like amphibole, which contain a hydroxide ion (OH–) that is derived from water, but is missing a hydrogen ion (H+). The calcium sulfate without water is a different mineral than gypsum called anhydrite (CaSO4).
3.4.4 Phosphates

Phosphate minerals have a tetrahedral phosphate unit (PO4-3) combined with various anions and cations. In some cases arsenic or vanadium can substitute for phosphorus. Phosphates are an important ingredient of fertilizers as well as detergents, paint, and other products. The best known phosphate mineral is apatite, Ca5(PO4)3(F,Cl,OH), variations of which are found in teeth and bones. The gemstone turquoise [CuAl6(PO4)4(OH)8·4H2O ] is a copper-rich phosphate mineral that, like gypsum, contains water molecules.
3.4.5 Native Element Minerals


Native element minerals, usually metals, occur in nature in a pure or nearly pure state. Gold is an example of a native element mineral; it is not very reactive and rarely bonds with other elements so it is usually found in an isolated or pure state. The non-metallic and poorly-reactive mineral carbon is often found as a native element, such as graphite and diamonds. Mildly reactive metals like silver, copper, platinum, mercury, and sulfur sometimes occur as native element minerals. Reactive metals such as iron, lead, and aluminum almost always bond to other elements and are rarely found in a native state.
[ays_quiz id=”20″]
3.5 Identifying Minerals

Geologists identify minerals by their physical properties. In the field, where geologists may have limited access to advanced technology and powerful machines, they can still identify minerals by testing several physical properties: luster and color, streak, hardness, crystal habit, cleavage and fracture, and some special properties. Only a few common minerals make up the majority of Earth’s rocks and are usually seen as small grains in rocks. Of the several properties used for identifying minerals, it is good to consider which will be most useful for identifying them in small grains surrounded by other minerals.
3.5.1 Luster and Color
![Source: By John Chapman (Pyrope) (Own work) [<a href="http://www.gnu.org/copyleft/fdl.html">GFDL</a> or <a href="http://creativecommons.org/licenses/by-sa/4.0-3.0-2.5-2.0-1.0">CC BY-SA 4.0-3.0-2.5-2.0-1.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AMolly_Hill_molybdenite.JPG">via Wikimedia Commons</a> The crystal looks like metal.](https://opengeology.org/textbook/wp-content/uploads/2016/07/Molly_Hill_molybdenite-300x225.jpg)

Nonmetallic luster doesn’t look like a metal and may be described as vitreous (glassy), earthy, silky, pearly, and other surface qualities. Nonmetallic minerals may be shiny, although their vitreous shine is different from metallic luster. See the table for descriptions and examples of nonmetallic luster.
| Luster | Image | Description |
|---|---|---|
| Vitreous/glassy | ![By Didier Descouens (Own work) [<a href="http://creativecommons.org/licenses/by-sa/4.0">CC BY-SA 4.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AQuartz_Br%C3%A9sil.jpg">via Wikimedia Commons</a> A mass of quartz crystals showing typical six sided habit with points](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.23_-Quartz_Br%C3%A9sil-150x150.jpg) |
Surface is shiny like glass |
| Earthy/dull | Dull, like dried mud or clay | |
| Silky | ![By Ra'ike (see also: de:Benutzer:Ra'ike) (Own work) [<a href="http://www.gnu.org/copyleft/fdl.html">GFDL</a>, <a href="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0</a> or <a href="http://creativecommons.org/licenses/by/2.5">CC BY 2.5</a>], <a href="https://commons.wikimedia.org/wiki/File%3ASelenite_Gips_Marienglas.jpg">via Wikimedia Commons</a> Specimen showing silky luster](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.48_silky_luster_Selenite_Gips_Marienglas-1-300x230.jpg) |
Soft shine like silk fabric |
| Pearly | ![By Luis Miguel Bugallo Sánchez (Lmbuga Commons)(Lmbuga Galipedia)Publicada por/Publish by: Luis Miguel Bugallo Sánchez (Own work) [<a href="http://www.gnu.org/copyleft/fdl.html">GFDL</a> or <a href="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AMineral_Mica_GDFL006.JPG">via Wikimedia Commons</a> Specimen showing pearly luster like the inside of a clam shell](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.49_pearly_luster_Mineral_Mica_GDFL006-300x218.jpg) |
Like the inside of a clam shell or mother-of-pearl |
| Submetallic | ![By Andreas Früh (Andel) (Own work) [<a href="http://www.gnu.org/copyleft/fdl.html">GFDL</a> or <a href="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ASphalerite4.jpg">via Wikimedia Commons</a> Photo of mineral exhibiting submetallic luster](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.44_submetallic_Sphalerite4-300x277.jpg) |
Has the appearance of dull metal, like pewter. These minerals would usually still be considered metallic. Submetallic appearance can occur in metallic minerals because of weathering. |
![Source: By Graeme Churchard from Bristol (51.4414, -2.5242), UK (In Melbourne MuseumUploaded by PDTillman) [<a href="http://creativecommons.org/licenses/by/2.0">CC BY 2.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AAzurite_in_siltstone%2C_Malbunka_mine_NT.jpg">via Wikimedia Commons</a> There are two dark blue disks on white siltstone.](https://opengeology.org/textbook/wp-content/uploads/2016/07/Azurite_in_siltstone_Malbunka_mine_NT-245x300.jpg)
Some minerals predominantly show a single color. Malachite and azurite are green and blue, respectively, because of their copper content. Other minerals have a predictable range of colors due to elemental substitutions, usually via a solid solution. Feldspars, the most abundant minerals in the earth’s crust, are complex, have solid solution series, and present several colors including pink, white, green, gray and others. Other minerals also come in several colors, influenced by trace amounts of several elements. The same element may show up as different colors, in different minerals. With notable exceptions, color is usually not a definitive property of minerals. For identifying many minerals. a more reliable indicator is streak, which is the color of the powdered mineral.
3.5.2 Streak

Streak examines the color of a powdered mineral, and can be seen when a mineral sample is scratched or scraped on an unglazed porcelain streak plate. A paper page in a field notebook may also be used for the streak of some minerals. Minerals that are harder than the streak plate will not show streak, but will scratch the porcelain. For these minerals, a streak test can be obtained by powdering the mineral with a hammer and smearing the powder across a streak plate or notebook paper.
While mineral surface colors and appearances may vary, their streak colors can be diagnostically useful. An example of this property is seen in the iron-oxide mineral hematite. Hematite occurs in a variety of forms, colors and lusters, from shiny metallic silver to earthy red-brown, and different physical appearances. A hematite streak is consistently reddish brown, no matter what the original specimen looks like. Iron sulfide or pyrite, is a brassy metallic yellow. Commonly named fool’s gold, pyrite has a characteristic black to greenish-black streak.
3.5.3 Hardness
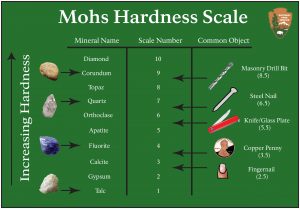
Hardness measures the ability of a mineral to scratch other substances. The Mohs Hardness Scale gives a number showing the relative scratch-resistance of minerals when compared to a standardized set of minerals of increasing hardness. The Mohs scale was developed by German geologist Fredrick Mohs in the early 20th century, although the idea of identifying minerals by hardness goes back thousands of years. Mohs hardness values are determined by the strength of a mineral’s atomic bonds.
The figure shows the minerals associated with specific hardness values, together with some common items readily available for use in field testing and mineral identification. The hardness values run from 1 to 10, with 10 being the hardest; however, the scale is not linear. Diamond defines a hardness of 10 and is actually about four times harder than corundum, which is 9. A steel pocketknife blade, which has a hardness value of 5.5, separates between hard and soft minerals on many mineral identification keys.
3.5.4 Crystal Habit
Minerals can be identified by crystal habit, how their crystals grow and appear in rocks. Crystal shapes are determined by the arrangement of the atoms within the crystal structure. For example, a cubic arrangement of atoms gives rise to a cubic-shaped mineral crystal. Crystal habit refers to typically observed shapes and characteristics; however, they can be affected by other minerals crystallizing in the same rock. When minerals are constrained so they do not develop their typical crystal habit, they are called anhedral. Subhedral crystals are partially formed shapes. For some minerals characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in rock. An example is garnet. Minerals grown freely where the crystals are unconstrained and can take characteristic shapes often form crystal faces. A euhedral crystal has a perfectly formed, unconstrained shape. Some minerals crystallize in such tiny crystals, they do not show a specific crystal habit to the naked eye. Other minerals, like pyrite, can have an array of different crystal habits, including cubic, dodecahedral, octahedral, and massive. The table lists typical crystal habits of various minerals.
| Habit | Image | Examples |
|---|---|---|
| Bladed
long and flat crystals |
![By User:Aelwyn (Self made picture) [<a href="http://www.gnu.org/copyleft/fdl.html">GFDL</a>, <a href="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0</a> or <a href="http://creativecommons.org/licenses/by-sa/2.5-2.0-1.0">CC BY-SA 2.5-2.0-1.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AKyanite_crystals.jpg">via Wikimedia Commons</a> The crystals are long and rectangular](https://opengeology.org/textbook/wp-content/uploads/2016/07/Kyanite_crystals-300x225.jpg) |
kyanite, amphibole, gypsum |
| Botryoidal/mammillary
blobby, circular crystals |
![By Didier Descouens (Own work) [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AMalachite_Kolwezi_Katanga_Congo.jpg">via Wikimedia Commons</a> The mineral is bulbous](https://opengeology.org/textbook/wp-content/uploads/2016/07/Malachite_Kolwezi_Katanga_Congo-150x150.jpg) |
hematite, malachite, smithsonite |
| Coating/laminae/druse
crystals that are small and coat surfaces |
![By Juppi66 (Juppi66) [Public domain], <a href="https://commons.wikimedia.org/wiki/File%3AAmetyst-geode.jpg">via Wikimedia Commons</a> The rock is hollowed and filled with purple minerals](https://opengeology.org/textbook/wp-content/uploads/2016/07/Ametyst-geode-150x150.jpg) |
quartz, calcite, malachite, azurite |
| Cubic
cube-shaped crystals |
pyrite, galena, halite | |
| Dodecahedral
12-sided polygon shapes |
![By Didier Descouens (Own work) [<a href="http://creativecommons.org/licenses/by-sa/4.0">CC BY-SA 4.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3APyrite_elbe.jpg">via Wikimedia Commons</a> Crystals of pyrite showing dodecahedral habit](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.53_habit_dodecahedral_Pyrite_elbe-300x195.jpg) |
garnet, pyrite |
| Dendritic
branching crystals |
![By Wilson44691 [Public domain], <a href="https://commons.wikimedia.org/wiki/File%3ADendrites01.jpg">via Wikimedia Commons</a> The mineral look like a fern. They are black and branching.](https://opengeology.org/textbook/wp-content/uploads/2016/07/Dendrites01-150x150.jpg) |
Mn-oxides, copper, gold |
| Equant
crystals that do not have a long direction |
![By S kitahashi assumed (based on copyright claims). [<a href="http://www.gnu.org/copyleft/fdl.html">GFDL</a>, <a href="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0</a> or <a href="http://creativecommons.org/licenses/by-sa/2.5">CC BY-SA 2.5</a>], <a href="https://commons.wikimedia.org/wiki/File%3APeridot2.jpg">via Wikimedia Commons</a> The crystal is light green.](https://opengeology.org/textbook/wp-content/uploads/2016/07/Peridot2-150x150.jpg) |
olivine, garnet, pyroxene |
| Fibrous
thin, very long crystals |
![By Didier Descouens (Own work) [<a href="http://creativecommons.org/licenses/by-sa/4.0">CC BY-SA 4.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ATremolite_Campolungo.jpg">via Wikimedia Commons</a> It is white and fiberous](https://opengeology.org/textbook/wp-content/uploads/2016/07/Tremolite_Campolungo-150x150.jpg) |
serpentine, amphibole, zeolite |
| Layered, sheets
stacked, very thin, flat crystals |
mica (biotite, muscovite, etc.) | |
| Lenticular/platy
crystals that are plate-like |
![Rob Lavinsky, <a rel="nofollow" class="external text" href="http://www.irocks.com/">iRocks.com</a> – CC-BY-SA-3.0 [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ACalcite-Wulfenite-tcw15b.jpg">via Wikimedia Commons</a> The orange wulfenite is platey](https://opengeology.org/textbook/wp-content/uploads/2016/07/Calcite-Wulfenite-tcw15b-150x150.jpg) |
selenite roses, wulfenite, calcite |
| Hexagonal
crystals with six sides |
quartz, hanksite, corundum | |
| Massive/granular
Crystals with no obvious shape, microscopic crystals |
limonite, pyrite, azurite, bornite | |
| Octahedral
4-sided double pyramid crystals |
![Source: Fluorite_crystals_270x444.jpg: Ryan Salsburyderivative work: TCO (Fluorite_crystals_270x444.jpg) [<a href="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0</a> or <a href="http://www.gnu.org/copyleft/fdl.html">GFDL</a>], <a href="https://commons.wikimedia.org/wiki/File%3AFluorite_crystals_(rotated_90).jpg">via Wikimedia Commons</a> Perfedt octahedral cleavage in fluorite generates octagon-shaped cleavage flakes.](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.62_octahedral_cleavage_Fluorite_crystals_rotated_90-300x182.jpg) |
diamond, fluorite, magnetite, pyrite |
| Prismatic/columnar
very long, cylindrical crystals |
![Rob Lavinsky, <a rel="nofollow" class="external text" href="http://www.irocks.com/">iRocks.com</a> – CC-BY-SA-3.0 [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ATourmaline-22335.jpg">via Wikimedia Commons</a> The mineral is a long cylinder.](https://opengeology.org/textbook/wp-content/uploads/2016/07/Tourmaline-150x150.jpg) |
tourmaline, beryl, barite |
| Radiating
crystals that grow from a point and fan out |
![Rob Lavinsky, <a rel="nofollow" class="external text" href="http://www.irocks.com/">iRocks.com</a> – CC-BY-SA-3.0 [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3APyrophyllite-236595.jpg">via Wikimedia Commons</a> The mineral is orange](https://opengeology.org/textbook/wp-content/uploads/2016/07/Pyrophyllite-236595-150x150.jpg) |
pyrite “suns”, pyrophyllite |
| Rhombohedral
crystals shaped like slanted cubes |
calcite, dolomite | |
| Tabular/blocky/stubby
sharp-sided crystals with no long direction |
![Rob Lavinsky, <a rel="nofollow" class="external text" href="http://www.irocks.com/">iRocks.com</a> – CC-BY-SA-3.0 [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ADiopside-172005.jpg">via Wikimedia Commons</a> Dark green crystals of diopside, a member of the pyroxene family](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.14_Diopside-172005-150x150.jpg) |
feldspar, pyroxene, calcite |
| Tetrahedral
three-sided, pyramid-shaped crystals |
![Rob Lavinsky, <a rel="nofollow" class="external text" href="http://www.irocks.com/">iRocks.com</a> – CC-BY-SA-3.0 [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ATetrahedrite-Chalcopyrite-Sphalerite-251531.jpg">via Wikimedia Commons</a> The dark brown mineral is triangular](https://opengeology.org/textbook/wp-content/uploads/2016/07/Tetrahedrite-Chalcopyrite-Sphalerite-251531-150x150.jpg) |
magnetite, spinel, tetrahedrite |
![Scource: Rob Lavinsky, <a rel="nofollow" class="external text" href="http://www.irocks.com/">iRocks.com</a> – CC-BY-SA-3.0 [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AStaurolite-62645.jpg">via Wikimedia Commons</a> The brown minerals are replicated in different directions](https://opengeology.org/textbook/wp-content/uploads/2016/07/Staurolite-62645-150x150.jpg)
![Source: Rob Lavinsky, <a rel="nofollow" class="external text" href="http://www.irocks.com/">iRocks.com</a> – CC-BY-SA-3.0 [<a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AGypsum-235288.jpg">via Wikimedia Commons</a> The mineral has many parallel lines on it](https://opengeology.org/textbook/wp-content/uploads/2016/07/GypsumStriations-150x150.jpg)

Striations and twinning are related properties in some minerals including plagioclase feldspar. Striations are optical lines on a cleavage surface. Because of twinning in the crystal, striations show up on one of the two cleavage faces of the plagioclase crystal.
3.5.5 Cleavage and Fracture
Minerals often show characteristic patterns of breaking along specific cleavage planes or show characteristic fracture patterns. Cleavage planes are smooth, flat, parallel planes within the crystal. The cleavage planes may show as reflective surfaces on the crystal, as parallel cracks that penetrate into the crystal, or show on the edge or side of the crystal as a series of steps like rice terraces. Cleavage arises in crystals where the atomic bonds between atomic layers are weaker along some directions than others, meaning they will break preferentially along these planes. Because they develop on atomic surfaces in the crystal, cleavage planes are optically smooth and reflect light, although the actual break on the crystal may appear jagged or uneven. In such cleavages, the cleavage surface may appear like rice terraces on a mountainside that all reflect sunlight from a particular sun angle. Some minerals have a strong cleavage, some minerals only have weak cleavage or do not typically demonstrate cleavage.

For example, quartz and olivine rarely show cleavage and typically break into conchoidal fracture patterns.
Graphite has its carbon atoms arranged into layers with relatively strong bonds within the layer and very weak bonds between the layers. Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating quality.
Mineral fracture surfaces may be rough and uneven or they may be show conchoidal fracture. Uneven fracture patterns are described as irregular, splintery, fibrous. A conchoidal fracture has a smooth, curved surface like a shallow bowl or conch shell, often with curved ridges. Natural volcanic glass, called obsidian, breaks with this characteristic conchoidal pattern
![Source: By Modris Baum (http://www.mindat.org/photo-458851.html) [Public domain], <a href="https://commons.wikimedia.org/wiki/File%3AArgentiferous_Galena-458851.jpg">via Wikimedia Commons</a> Specimen of galena showing cubic cleavage](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.64_galena_cleavage_Argentiferous_Galena-458851-300x261.jpg)

In some minerals, distinguishing cleavage planes from crystal faces may be challenging for the student. Understanding the nature of cleavage and referring to the number of cleavage planes and cleavage angles on identification keys should provide the student with enough information to distinguish cleavages from crystal faces. Cleavage planes may show as multiple parallel cracks or flat surfaces on the crystal. Cleavage planes may be expressed as a series of steps like terraced rice paddies. See the cleavage surfaces on galena above or plagioclase below. Cleavage planes arise from the tendency of mineral crystals to break along specific planes of weakness within the crystal favored by atomic arrangements. The number of cleavage planes, the quality of the cleavage surfaces, and the angles between them are diagnostic for many minerals and cleavage is one of the most useful properties for identifying minerals. Learning to recognize cleavage is an especially important and useful skill in studying minerals.

![Source: By No machine-readable author provided. Zimbres assumed (based on copyright claims). [<a href="http://creativecommons.org/licenses/by-sa/2.5">CC BY-SA 2.5</a>], <a href="https://commons.wikimedia.org/wiki/File%3AAmphibol.jpg">via Wikimedia Commons</a> Photomicrograph showing 120/60 degree cleavage in amphibole](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.63_cleavage_in_Amphibole-295x300.jpg)
Cleavages on common rock-forming minerals
- Quartz—none (conchoidal fracture)
- Olivine—none (conchoidal fracture)
- Mica—1 perfect
- Feldspar—2 perfect at 90°
- Pyroxene—2 imperfect at 90°
- Amphibole—2 perfect at 60°/120°
- Calcite—3 perfect at approximately 75°
- Halite, galena, pyrite—3 perfect at 90°
3.5.6 Special Properties
![By / Zeichner: Sec (Self-photographed) [Public domain], via Wikimedia Commons The words on the page are projected upwards onto the mineral](https://opengeology.org/textbook/wp-content/uploads/2016/07/Ulexit_Fernsehstein-150x150.jpg)
![I, <a href="/wiki/User:Gump_Stump" title="User:Gump Stump">Gump Stump</a> [<a href="http://www.gnu.org/copyleft/fdl.html">GFDL</a> or <a href="http://creativecommons.org/licenses/by-sa/3.0">CC BY-SA 3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ALatrobe_gold_nugget_Natural_History_Museum.jpg">via Wikimedia Commons</a> The nugget is gold](https://opengeology.org/textbook/wp-content/uploads/2016/07/Latrobe_gold_nugget_Natural_History_Museum-225x300.jpg)
A simple test for identifying calcite and dolomite is to drop a bit of dilute hydrochloric acid (10-15% HCl) on the specimen. If the acid drop effervesces or fizzes on the surface of the rock, the specimen is calcite. If it does not, the specimen is scratched to produce a small amount of powder and test with acid again. If the acid drop fizzes slowly on the powdered mineral, the specimen is dolomite. The difference between these two minerals can be seen in the video. Geologists who work with carbonate rocks carry a small dropper bottle of dilute HCl in their field kit. Vinegar, which contains acetic acid, can be used for this test and is used to distinguish non-calcite fossils from limestone. While acidic, vinegar produces less of a fizzing reaction because acetic acid is a weaker acid.
![Source: By Ryan Somma (Magnetite Lodestone) [<a href="http://creativecommons.org/licenses/by-sa/2.0">CC BY-SA 2.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AMagnetite_Lodestone.jpg">via Wikimedia Commons</a> The paperclip is sticking up into the air.](https://opengeology.org/textbook/wp-content/uploads/2016/07/Magnetite_Lodestone-150x150.jpg)
![Source: By Mike Beauregard from Nunavut, Canada (Eyeful of Sky) [<a href="http://creativecommons.org/licenses/by/2.0">CC BY 2.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3AEyeful_of_Sky_(5604800064).jpg">via Wikimedia Commons</a> Striations or parallel dark lines on one cleavage surface on plagioclase feldspar](https://opengeology.org/textbook/wp-content/uploads/2016/07/03.65_Striations_in_plagioclase-300x242.jpg)

Striations on mineral cleavage faces are an optical property that can be used to separate plagioclase feldspar from potassium feldspar (K-spar). A process called twinning creates parallel zones in the crystal that are repeating mirror images. The actual cleavage angle in plagioclase is slightly different than 90o and the alternating mirror images in these twinned zones produce a series of parallel lines on one of plagioclase’s two cleavage faces. Light reflects off these twinned lines at slightly different angles which then appear as light and dark lines called striations on the cleavage surface. Potassium feldspar does not exhibit twinning or striations but may show linear features called exsolution lamellae, also known as perthitic lineation or simply perthite. Because sodium and potassium do not fit into the same feldspar crystal structure, the lines are created by small amounts of sodium feldspar (albite) separating from the dominant potassium feldspar (K-spar) within the crystal structure. The two different feldspars crystallize out into roughly parallel zones within the crystal, which are seen as these linear markings.

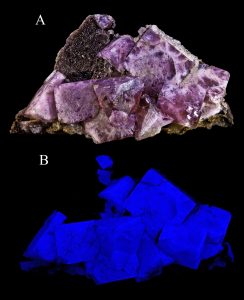
One of the most interesting special mineral properties is fluorescence. Certain minerals, or trace elements within them, give off visible light when exposed to ultraviolet radiation or black light. Many mineral exhibits have a fluorescence room equipped with black lights so this property can be observed. An even rarer optical property is phosphorescence. Phosphorescent minerals absorb light and then slowly release it, much like a glow-in-the-dark sticker.
[ays_quiz id=”21″]
Summary
Minerals are the building blocks of rocks and essential to understanding geology. Mineral properties are determined by their atomic bonds. Most minerals begin in a fluid, and either crystallize out of cooling magma or precipitate as ions and molecules out of a saturated solution. The silicates are largest group of minerals on Earth, by number of varieties and relative quantity, making up a large portion of the crust and mantle. Based on the silicon-oxygen tetrahedra, the crystal structure of silicates reflects the fact that silicon and oxygen are the top two of Earth’s most abundant elements. Non-silicate minerals are also economically important, and providing many types of construction and manufacturing materials. Minerals are identified by their unique physical properties, including luster, color, streak, hardness, crystal habit, fracture, cleavage, and special properties.
[ays_quiz id=”22″]
References
- Clarke, F.W.H.S.W., 1927, The Composition of the Earth’s Crust: Professional Paper, United States Geological Survey, Professional Paper.
- Gordon, L.M., and Joester, D., 2011, Nanoscale chemical tomography of buried organic-inorganic interfaces in the chiton tooth: Nature, v. 469, no. 7329, p. 194–197.
- Hans Wedepohl, K., 1995, The composition of the continental crust: Geochim. Cosmochim. Acta, v. 59, no. 7, p. 1217–1232.
- Lambeck, K., 1986, Planetary evolution: banded iron formations: v. 320, no. 6063, p. 574–574.
- metallic bond | chemistry.
- Scerri, E.R., 2007, The Periodic Table: Its Story and Its Significance: Oxford University Press, USA.
- Thomson, J.J., 1897, XL. Cathode Rays: Philosophical Magazine Series 5, v. 44, no. 269, p. 293–316.
- Trenn, T.J., Geiger, H., Marsden, E., and Rutherford, E., 1974, The Geiger-Marsden Scattering Results and Rutherford’s Atom, July 1912 to July 1913: The Shifting Significance of Scientific Evidence: Isis, v. 65, no. 1, p. 74–82.