
Callan Bentley photo.
LEARNING OBJECTIVES
After reading this chapter, students should be able to:
- Summarize the physical and chemical conditions necessary for the origins of life
- Describe early, unicellular forms of life on Earth
- Explain how life diversifies into multicellular forms
- Summarize the highlights of skeletonized and land-based life forms over time
Introduction
Based on current scientific knowledge, Earth is unique in its ability to host life in the diverse array seen throughout geologic time. With the processes of evolution as constraint and guide, life has developed numerous innovations that have allowed organisms of various kinds to make use of the available resources on this planet. This chapter explores the impact on some of these innovations to better understand the history of life on Earth.
In the Beginning
Conditions for first life

No one can say exactly when and where life began on Earth, but it is certain that the origin of life is one of the most critical, planet-changing events ever to occur. Based on scientific observation and reasoning, theories have been developed to explain the ideal conditions that allowed life to start. Conventional scientific thought suggests that extreme heat from formational, early meteorite bombardment of Earth during the Hadean would have made conditions at the surface too harsh for life to begin–literally, portions of the early crust melted from these collisions (e.g., Marchi et al., 2014). However, despite continued impacts during the Late Heavy Bombardment, some studies that suggest only a fraction of the planet’s crust would have melted, leaving potential areas of refuge deep in the crust or within hydrothermal vents at the bottom of the oceans. Based on geochemical data, oceans are thought to have developed very early in Earth’s history, by about 4.3 billion years ago (i.e., during the early Hadean). Thus, while the possibility exists that life could have arisen during the Hadean, given the minimal availability of rocks from this interval, scientists in search of first life are typically working in rocks of Archean age.

Finding definitive evidence of the first life forms on Earth presents a challenge. While there are geochemical signatures to many early Archean rocks that suggest organisms may have been present, the organisms would be microscopic cells with no hard parts. Additionally, most of the Archean rocks that potentially preserve these fossils have been subjected to well over 3 billion years of tectonics and weathering. Schopf (1993) proposed they had discovered the oldest known fossils in the 3.5 billion year old (Archean) Apex Chert of western Australia. Debate quickly ensued in peer-reviewed literature, and many geoscientists now interpret the filamentous structures from the Apex Chert are the result of mineral growth around hydrothermal vents (e.g., Marshall et al., 2011). Currently, sulfur-metabolizing microfossils in the 3.4 billion year old (Archean) hydrothermal cherts of the Strelley Pool Formation of western Australia are considered the oldest definitive organic microfossils (Wacey et al., 2011; Alleon et al., 2018). However, Dodd et al. (2017) showed evidence of early life as tubular and filamentous structures near hydrothermal vent deposits of the 3.77-4.28 billion year old (Archean) rocks of the Nuvvuagittuq Belt in Quebec, Canada. While all of these findings continue to be examined in further detail, there is a common thread–early life seems to have begun in association with the warm, chemically enriched environments of hydrothermal vents.

Today, hydrothermal vents are found in the deep ocean along mid-oceanic ridges at divergent plate boundaries and at volcanic hot spots. In these environments, cold water from the bottom of the ocean seeps along fissures found at the plate boundary. While underground, the water is heated by and mixes with fluids released from the rising magma. The super-heated water is able to carry many dissolved minerals and mobile ions. These ions are carried along with the water to the seafloor, and are then precipitated as mineral deposits around the vents. Sometimes these mineral deposits are infused into the surrounding deep sea sediments, and sometimes they build impressive “chimneys” of towering rock. Sulfur-based minerals are particularly common near the vents. The vents are surprisingly diverse in life, with sulfur-reducing bacteria extracting energy from compounds in the vented fluids. Using the principle of uniformitarianism, the first organisms at Hadean hydrothermal vents were therefore also likely sulfur-reducing bacteria. These bacteria were chemoautotrophs, meaning they extract the energy necessary for cellular functions from inorganic compounds. In the case of sulfur-reducing bacteria, they “breathe” sulfate (SO42-), and reduce sulfur to hydrogen sulfide (H2S). Sulfur-reducing bacteria are found today at the Mid-Atlantic Ridge, East Pacific Rise, and in pools associated with land-based hydrothermal activity like the hot springs and geysers of Yellowstone National Park in Wyoming. Some sulfur-reducing bacteria are also found in coastal environments–if you have ever noticed a rotten-egg odor in salt marshes, you’ve smelled the scent of chemoautotroph productivity! These prokaryotes belong to the Domain Archaea, which includes a diverse array of microbes that are able to live in chemically harsh conditions over a wide range of temperatures.
The prokaryotic pioneers
So the first life forms on Earth were likely sulfur-reducing bacteria, which are prokaryotes. Prokaryotes are structurally rather simple–they are single-celled, small in size (0.1 – 10.0 μm), and have no nucleus or other organelles. Make no mistake though, these simple organisms have changed the trajectory of Earth’s history in major ways. To quote Shakespeare, “Though she be but little, she is fierce!”
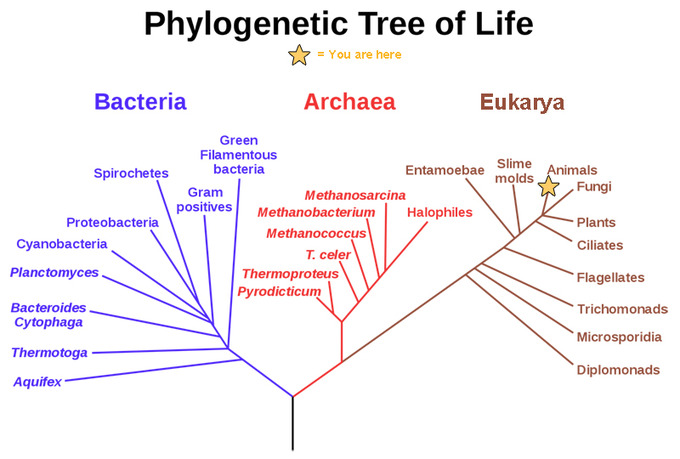
Two of the three Domains of life, Archaea and Bacteria, are prokaryotes. The third Domain, Eukarya, to which you belong, is discussed later in this chapter. Prokaryotes live on and in just about everything on Earth. They have been found in sulfuric volcanic lakes, hypersaline springs, glacial ice, growing on crystals deep in caves, and under rocks at the tops of mountains. In the human body, they outnumber human cells by 3 to 1. Rough estimates of modern oceanic prokaryotic diversity suggest more than 2 million species exist, which does not even include all the possible diversity on land. Consider how quickly bacteria evolve–check out this video that shows bacterial mutations against antibiotics in just 11 days! Then, remember that prokaryotes have been evolving for 3.5 billion years–there is no way the true diversity of prokaryotes will ever be known!

In many rocks of similar age as those preserving the Archean sulfur-reducing bacteria, macroscopic sedimentary structures known as stromatolites record the history of another important group–the cyanobacteria. Stromatolites are layered structures created when mats of cyanobacteria are smothered by fine calcite mud distributed by wave action in shallow marine environments. The bacteria grow through the mud layer to create a new mat, and the process repeats, producing a thinly laminated structure that is visible when lithified. Nutman et al. (2016) suggested they had found stromatolites in Greenland that were 3.7 billion years old (Archean), but this has been disputed by Allwood et al. (2018). The most widely accepted definitive stromatolites are those in the 3.5 billion year old (Archean) Dresser Formation in Australia.
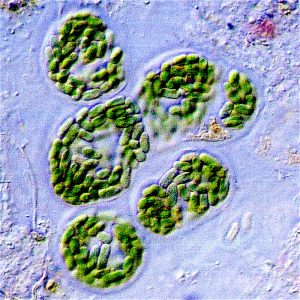
Cyanobacteria are photoautotrophs, meaning that they require sunlight to create their own food via photosynthesis. Therefore, these organisms are found in the shallow, photic zone of marine environments where sunlight can readily penetrate the water. Photosynthesis uses carbon dioxide (CO2) as a source of carbon for cellular functions, and produces free oxygen (O2) as a waste product. Cyanobacteria were the dominant life form on Earth for about 2 billion years, spanning the Archean and a major portion of the Proterozoic. As a result of all that photosynthesis, the amount of O2 in Earth’s atmosphere significantly increased over time in one of the most slow-building (but literally life-changing) events of all-time–the Great Oxidation Event. Banded iron formations (BIFs) are first preserved in the rock record around 2.4 billion years ago (Archean). BIFs consist of alternating layers of dark grey to black iron oxides (hematite and magnetite) with red to yellow chert.

The interpretation of BIFs is that excess oxygen created by the photosynthesizing cyanobacteria in shallow oceans was available to combine with iron ions in seawater to produce the dark oxidized iron layers. The source of iron is likely a combination of both ions released from hydrothermal vents as well as input from chemical weathering of mafic-rich crustal rock from land. These distinct chemical sedimentary rocks were globally pervasive until about 1.8 billion years ago (Paleoproterozoic). BIFs decline in the rock record as the amount of oxygen in the atmosphere continued to climb. With more atmospheric oxygen available, iron ions were oxidized in terrestrial settings before they were transported to the oceans.
Eukaryotes: one small step for cells, one giant leap for life
A consistent pattern that we see in the rock record is that life modifies Earth systems, and Earth systems, in turn, modify life. The accumulation of oxygen in the atmosphere due to prokaryotic photosynthesis ultimately created a new resource that life could exploit as a source of energy. Around 1.8 billion years ago (Paleoproterozoic), definitive eukaryotes appear in the fossil record, armed with a new method of making energy for cellular function–aerobic respiration. Aerobic respiration requires oxygen to produce molecules of ATP which can then be stored and metabolized when needed to power a cell.
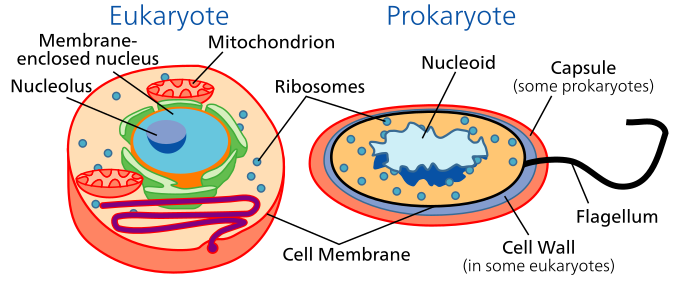
Eukaryotic cells contain a membrane-bound nucleus that houses the cell’s DNA and a variety of other organelles that assist with cell activities. In particular, mitochondria are important organelles because they are associated with ATP production within all eukaryotic cells. In plants, chloroplasts are also necessary as the sites where photosynthesis occurs within the cell. Because they contain a variety of organelles, eukaryotic cells are generally one to two orders of magnitude larger than prokaryotic cells (eukaryotes are typically 10 – 100 μm).
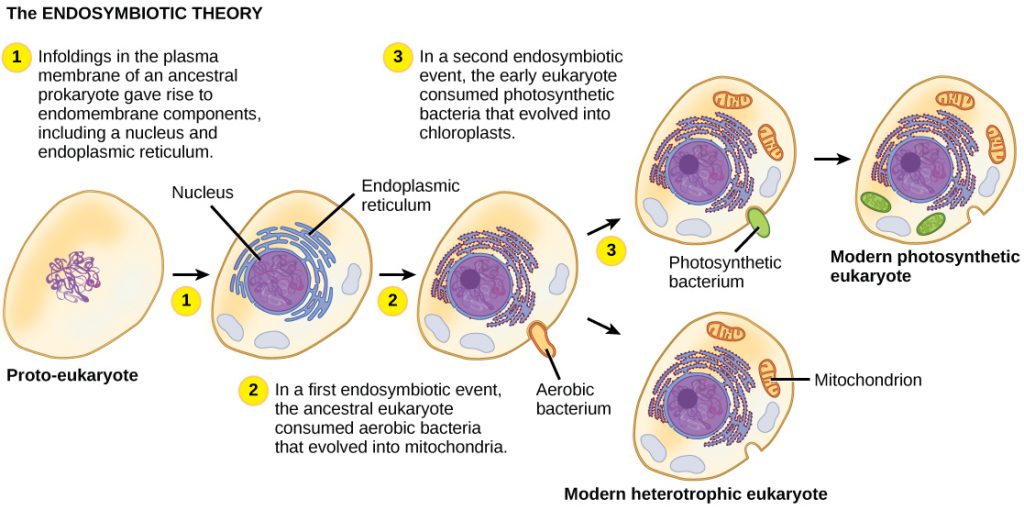
So, how did eukaryotic cells first evolve? Current science supports endosymbiosis theory (ET), which states that eukaryotic cells arose due to a mutually beneficial relationship where one prokaryote lives inside another. For example, one prokaryote engulfed another but did not digest it, allowing both cells to persist. The host cell offered protection, and the ingested cell provided energy to allow both cells to function. Over time, the cells co-evolved, and became reliant on each other as a single system: a cell with energy-producing organelles, mitochondria and chloroplasts. ET was first championed by Lynn Margulis in the 1960s, but took decades to be more widely accepted. One of the major lines of evidence in support of ET is that both mitochondria and chloroplasts possess their own DNA, with genetic signatures very similar to modern prokaryotes. Additionally, organisms without mitochondria, like the amoeba Pelomyxa, instead host symbiotic bacteria that serve the same function as mitochondria, but are not yet so wedded to their host that they cannot survive on their own.
Today, eukaryotes include a wide variety of single-celled organisms, both with and without shells, like amoeba, foraminifera, coccolithophores, radiolaria, and diatoms. These are called protists. We classify them within the Kingdom Protista. All multicellular organisms are also eukaryotes, including all fungi, plants, and animals (Kingdoms Fungi, Plantae, and Animalia, respectively). The most widely accepted fossils that are interpreted as representing the first eukaryotes are acritarchs, a group of single-celled organisms that appear in the record about 1.8 billion years ago (Paleoproterozoic), well into the prokaryotic-driven Great Oxidation Event. Acritarchs are of unknown affinity, which means there is uncertainty as to where they fit on the tree of life. Acritarchs consist of a spherical sac-shaped cell ranging in size from 1.0 – 1000.0 μm, making them significantly larger than prokaryotes. The oldest forms are most commonly thought to be related to marine algae or dinoflagellates (a protist group). Also around 1.8 billion years ago (Paleoproterozoic), another fossil, Grypania spiralis, appears in the fossil record. This filamentous, spiral shaped fossil is commonly considered to be eukaryotic, primarily due to its size, leading some researchers adamantly debate that Grypania may also represent one of the first multicellular organisms on Earth.
Out onto the multicellular branches of life: the Ediacaran biota
Given that unicellular organisms had been quite successful for over a billion years, paleobiologists have wondered–what is the evolutionary benefit of a multicellular existence? Many scientists suggested that the typical pressures of natural selection, such as predation, could have played a role. In two interesting modern lab studies of unicellular green algae (Boraas et al., 1998; Herron et al., 2019), algal populations that grew into multicellular structures showed greater survival under predation. Multicellularity is likely an example of convergent evolution, occurring multiple times within different eukaryotic groups to create the diversity of multicellular eukaryotes that exist on Earth today.
Another potential evolutionary benefit shared by most multicellular organisms is the ability to reproduce sexually. Prokaryotes typically reproduce asexually by budding or fission, but the resulting organism is a genetic replicate unless a random mutation occurs. In the case of sexual reproduction, gametes (e.g., sperm and egg cells) recombine DNA to make genetically distinct offspring. Sexual reproduction does have certain disadvantages. For example, it takes a significantly longer time to reproduce and grow population sizes when only half the population (i.e., females) are capable of reproduction. However, the evolutionary trade-off from bringing together genes from two individuals can more rapidly produce advantageous traits and delete harmful mutations from a population. For example, the Red Queen hypothesis of sexual reproduction proposes that asexual organisms are more susceptible to parasitism due to their cloned DNA. Recombination of DNA through sex would allow an organism the genetic variability needed to defend against co-evolving parasites in the environment (Jokela et al., 2009). Given the incredible diversity of life on Earth, clearly both asexual and sexual reproduction are successful means to fully take advantages of Earth’s resources.

As with other forms of early life, the origins of multicellular life are difficult to pinpoint given the lack of hard parts available for preservation. The fossil record of the Ediacaran Period (635-541 million years ago) at the end of Neoproterozoic best captures this next major innovation of life. The Ediacaran biota are a diverse assemblage of complex, macroscopic multicellular body and trace fossils. This significant increase in diversity follows an extensive period of glaciation (Snowball Earth) during the Cryogenian (Neoproterozoic). Prokaryotes likely contributed to eukaryotic success; this biota would have originated during a time when oxygen levels in the surface oceans were still increasing due to photosynthesizing cyanobacteria. Recent geochemical research (Bekker et al., 2017) also indicates bacteria levels were high in the Ediacaran seas, creating higher concentrations of dissolved organic matter as a food resource for these organisms. The warmer, oxygen- and food-rich seas of the Ediacaran may have set the conditions necessary for multicellular life to globally flourish.
Ediacaran fossils have been found on every continent except Antarctica. They are typically preserved within sandstones of shallow marine settings, like the famous deposits from the White Sea Coast of Russia, the Flinders Range of Australia, and Namibia. In a few places, however, fossils of the biota have been found in finer-grained sediments of deeper waters, such as the ash beds at Mistaken Point in southeastern Newfoundland. The biota primarily consists of impressions (both casts and external molds) of soft-bodied organisms that do not usually preserve well in coarser, sandy sediments, and many are even found underlying turbidity current deposits, effectively smothered by the rapid burial. Some research suggests that the fine texture of pervasive microbial mats would aid in preservation of the fossil impressions.

The fossils reveal a few common shapes (also known as morphologies). Many are described as disc-shaped (Cyclomedusa, Mawsonites). Others, known as rangeomorphs, have a frond-like shape (Rangea, Charnia). Still others exhibit the first clear examples of bilateral symmetry (Kimberella). There are even some odd forms that have an apparent trilateral symmetry (Tribrachidium), which is unlike anything alive today. The coarse sediment in which these fossils are preserved has hidden much of their anatomic detail, thus where these organisms should be placed on the tree of life has been widely debated. Some researchers suggest the Ediacaran biota represents basal members of some certain invertebrate animal groups that take off in diversity during the subsequent Cambrian Explosion, such as cnidarians and mollusks. Others have argued for identification of various Ediacaran forms as giant protists, algae, worms, fungi, or in a phylum of their own, the Vendozoa. Recent chemical analyses of Dickinsonia fossils showed evidence of cholesteroids, molecular fossils of the compound cholesterol, which is only found in animal cells (Bobrovskiy et al., 2018). When compared to molecular analyses of the surrounding sediments, only stigmasteroids, molecular fossils indicative of the green algae of the microbial mats was found. This study supports the interpretation that at least some of the Ediacaran biota are some of the first true animals on Earth.
While many ichnotaxa from this time have been re-evaluated in recent years to be sedimentary structures or microbially related, most Ediacaran burrows are simple, horizontal trails and burrows, or rasping traces made in association with microbial mats. Rasping is a mode of feeding by scratching at a surface. Snails and sea urchins eat algae this way today. Toward the end of the Ediacaran, burrows begin to increase in complexity, with a shift to more vertical and branching shapes, suggesting animal motility was on the rise.
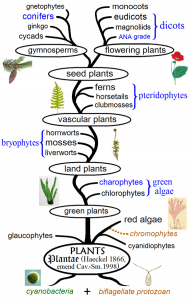
Phanerozoic Diversity of Life (According to the Hard Parts)
Before and after: the exoskeleton appears
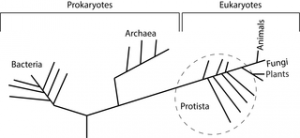
One of the most fascinating aspects of Earth history is the diversification of life through time. There are many ways to graphically represent this diversification. The “tree of life” diagram is one way to broadly represent phylogenetic relationships of the three major Domains of life, including the Archaea, Bacteria, and Eukarya. Within each of these main stems, further distinctions can be made as organisms are grouped with others that share similar physical traits, as discussed in the cladistics section of this textbook. However, one of the factors that is missing from the tree of life diagram is the aspect of time. As we have seen in the prior sections, it took a long time for life to separate into the three Domains, and even longer for Eukarya to split into the four Kingdoms: Protista, Fungi, Plantae, and Animalia. The next major innovation of life is so revolutionary that geoscientists literally draw a line in the sand(stone)–the Precambrian and everything after. During the Cambrian Period, which kicks off the Phanerozoic Eon, shelled organisms with an exoskeleton proliferate in the fossil record. This significant uptick in the diversity of marine life is known as the Cambrian Explosion. (Note there are small, shelled animals (metazoans) of uncertain affinities found in rocks of the Late Ediacaran known as the Tommotian fauna, including the genus Cloudina.)

Why did animals develop hard, mineralized exoskeletons in abundance during the Cambrian? It was likely that shells were the evolutionary answer to a biological need. Increased animal diversity at this time would lead to greater competition for resources, and an external shell offers several evolutionary advantages: it offers protection from physical and chemical variations in the environment, such as changes in water temperature and salinity; it offers protection from biological predators; and, it allows muscles additional mechanical leverage during locomotion, making it easier to burrow, for example. Being able to withstand seasonal shifts in currents, ward off predators, or exploit food sources below the seafloor would increase chances of survival. Interestingly, the boundary between the Proterozoic and Phanerozoic is marked by a shift in seawater chemistry that impacted carbonate mineral deposition in the oceans (Wood et al., 2017). During the Ediacaran (Neoproterozoic), dolomite (CaMg(CO3)2) was the dominant carbonate deposited in the oceans, but increased erosion of continental crustal rocks and chemical weathering led to an influx of calcium ions to the seas during the early stages of the Cambrian. Higher calcium levels allowed for easier precipitation of aragonite and calcite, the two polymorphs of CaCO3 that most marine organisms use to create their shells. As the Cambrian and Ordovician progressed, a wide array of body plans developed among representatives of most of today’s major invertebrate phyla, including sponges, corals, brachiopods, bryozoans, arthropods, annelids, mollusks, and echinoderms.
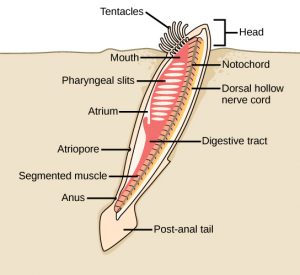
Internal skeletons, or endoskeletons, also have their origins in the Cambrian Period. The first endoskeletons were not mineralized bone, but rather, strong, flexible tissue known as cartilage. This is the same material that makes up shark skeletons and your own ears and nose today. Some of the first vertebrates are fossils from the early Cambrian Chengjiang locale in China. These vertebrates were marine organisms, eel-like in shape, with elongate, tapered bodies that possess a notochord and simple vertebrae. A notochord is a skeletal structure shared by all members of the phylum Chordata at some stage in their life cycle. The notochord helps to support muscles and define characteristic, mirroring, bilateral symmetry on either side of the chordate body’s midline. For many chordates, additional strength, flexibility and support are provided with the bones of the vertebral column. Note the spinal cord found in vertebrates is part of the nervous system, which is a completely different set of tissues from the notochord and vertebrae, which are part of the skeletal system.
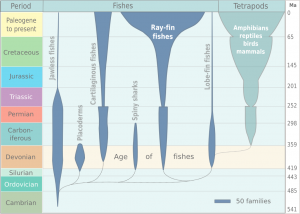
Throughout the Cambrian and into the Silurian, jawless fish (agnathans) and armored fish (ostracoderms) diversified. Interestingly, while the development of body external plates of the ostracoderms has traditionally been thought to be a classic response of protection to predation, there is also the added benefit of ion storage. In particular, bone stores phosphorous which is used by muscles for quick bursts of energy, like when swimming quickly away from predators as well. Jawed fish, including cartilaginous fish like sharks and rays (chondrichthyes), and bony fish (osteichthyes) appeared during the Silurian and radiated during the Devonian.
Here come the plants: landward, ho!
As human animals, we tend to focus on the parts of Earth history that tell our story; however, animals are not the only life form that moved to land during the Paleozoic, and in fact, plants beat our ancestors to the punch.

The first multicellular plants are marine green and red algaes that were likely derived from photosynthetic cyanobacteria during the Neoproterozoic. The first land plants evolved around the early Ordovician, when fossil evidence of the first bryophytes are found. Bryophytes include plants like mosses, hornworts, and liverworts, that are commonly found in moist areas. They do not rely on a root system for uptake of water and nutrients, rather taking these in via their leaves, and reproduce via spores. Later in the Ordovician, the first true vascular plants (trachaeophytes) evolved, and by the Devonian, this group looked like what you think of when you think of a plant–leafy and green, possessing a lignin-reinforced stem and roots that conduct water and nutrients through their tissues, and using seeds for reproduction.
Imagine land on Earth before plants. It would have been lots of bare rock, very much like deserts or areas after volcanic eruptions today. The first liverworts and mosses to colonize land would have begun to significantly impact physical and chemical weathering of terrestrial landscapes. Plants can assist in the development of soils from bedrock as they extract nutrients, retain water, and produce organic matter. Later, root systems of vascular plants could wedge into cracks in rock in search of water, physically creating more surface area for physical and chemical weathering to occur. However, given enough time and spread of populations, plants and their roots can also add stability to slopes, lead to the development of new ecological environments (forests, swamps), and provide new resources (leaves, seeds) for other life forms to put to evolutionary use.
Next up: invertebrate animals invade terrestrial environments
Invertebrate groups diversified significantly throughout the Paleozoic oceans (see diversity section below), increasing the competition for resources. As time went by, there was an increase in ecological tiering above and below the seafloor, as animals evolved forms that allow for deeper burrowing and higher extension away from the sediment-water interface. It was only a matter of time until life found a way to exploit the vast expanse of geographic space on land. This transition to land is thought to have occurred during the Devonian period, when global changes in oxygen levels in the ocean were in decline. This dysoxia was in part driven by excess runoff of organic matter from land due to dying plants, which triggered large scale algal and other plankton blooms in the oceans. An abundance of plankton uses up dissolved oxygen in the oceans, leaving less behind for the larger ocean dwellers. Today, “dead zones” develop at the mouths of modern rivers where concentrations of nutrients from over-fertilization of farms and other industrial processes create plankton blooms. This phenomenon ultimately may have been connected to the mass extinctions at the end of the Devonian.

It is important to recognize the transition to land by complex organisms was not instantaneous. Aquatic invertebrates would have had an advantage due to their exoskeletons, which allow for protection and moisture retention as these organisms adapted to freshwater ecosystems, like rivers and lakes, and eventually land. It is not surprising then that arthropods are the first aquatic invertebrate group to venture into the terrestrial realm. Out of all of the invertebrate phyla existing at the time, arthropods had the body plan with the greatest evolutionary flexibility–in particular, lots of segmented appendages that were highly specialized for different types of movement such as walking, burrowing, swimming, and feeding. Aquatic arthropods take in oxygen via book gills (a structure that looks similar to folded or overlapping sheets of paper) which are easily visible on horseshoe crabs you might find along the East Coast of the United States today. The first land arthropods would have developed internal book lungs from these structures, which we see in modern arachnids, like spiders and scorpions. Around the Late Silurian to Early Devonian, terrestrial fossils of tiny spider-like arthropods often found in association with plant fossils, scorpions, and myriapods (clade including millipedes, centipedes, eventually insects) have been found. In time, some groups of arthropods, including insects developed tracheae, a system of tube-like structures that directly deliver oxygen from external pores in the exoskeleton to tissues of the body. And so, the rest of us animals have come to live in a bug’s world.

Coevolution among insects and with other animals, coevolution with plants, adaptation to freshwater, and variable predation in larval versus adult forms are just some of the means by which insects have spread across the terrestrial realm. Perhaps most importantly though was the adaptation to the sky–arthropods are the only phyla of invertebrates to develop wings and flight. The first flying insects show up in the fossil record of the Carboniferous. While there is still debate in scientific circles, it is generally assumed wings represent another modification of appendages that were given a molecular and mechanical boost from high oxygen levels in muscles achieved via tracheae. The development of flight also corresponds to increases in atmospheric oxygen on a global level during the Carboniferous as vascular plants diversified, grew into large populations, and pumped out oxygen as a byproduct of photosynthesis. This rapid diversification of insects into all available ecological niches is known as an adaptive radiation, and this pattern is repeated by other terrestrial animal groups later in geologic time. Today, there are about one million described insect species, representing almost 75% of all known animals.
Vertebrates take their first steps
While arthropods are crawling and scurrying their way onto land, vertebrates were keeping an eye on their progress–literally! An interesting avenue of recent research suggests that vertebrates begin to show critical increases in eye size and a shift in eye position from the side to the top of the head during the interval leading up to their transition to land (MacIver et al., 2017). These researchers hypothesize that these changes in eyesight would have yielded better vision over longer distances, allowing these animals a better sightline to prey on land.
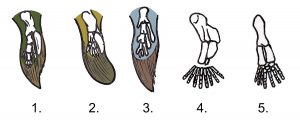
1. Tiktaalik. 2. Panderichthys. 3. Eusthenopteron. 4. Acanthostega.5. Ichthyostega ( hindleg ). Public domain image.
Other structural changes were also required to the vertebrate body plan to produce success on land. The first land-based animals are known collectively as tetrapods, referring to those animals moving about on four feet. Without the buoyancy provided by water, the bones of tetrapods would need to be more robust to provide more structural support to the body and allow for larger muscles to develop to aid in locomotion. Bony, lobe-finned fish (those in the sarcopterygian class) possess the basic vertebrate body plan in their appendages, and this same
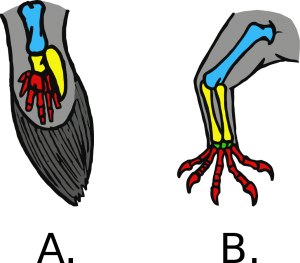
musculoskeletal template in their ancestors would have allowed for the evolution of muscular arms and legs. Specifically, their fins exhibit the same sequence of bones as all other tetrapod limbs: one bone at the point of body attachment, followed by a joint, two bones, another joint, then lots of little bones. Other changes are not particularly well-fossilized, but would also have been key to success on land: development of a three-chambered heart and more complex lungs to extract oxygen from air, continual modification to auditory systems to allow for better hearing in air, and development of more waterproof skin to retain tissue moisture.
Paleontologists who study the colonization of land are commonly looking for what are known as transitional fossils that show these gradual changes over time. One of the most famous transitional fossils of the Late Devonian is Tiktaalik, a fish discovered by a team of researchers headed by Neil Shubin and Ted Daeschler.
This fossil shows clear traits of being fish-like while also showing some of the necessary adaptations to being able to move about without the buoyancy of water. Tiktaalik possessed stronger limbs including hip-like structures on the hind limbs, a neck that allowed for swiveling motions, and large eye sockets on the top of the head. Interestingly, an increase in size and shift in the position of spiracles (skull openings that allow air to pass across a fish’s gills) toward the back and side of the skull in several transitional fossils (Eusthenopteron, Icthyostega, and Tiktaalik) suggest a positioning more in line with the location of lungs.
Tetrapods and plants: conquering land, sea, and air

The first tetrapods on land during the Devonian quickly evolve characteristics that are reminiscent of today’s amphibians, although modern amphibian groups generally evolved during the Triassic. While these animals began to spread into various environments on the continents as adults, there would be some limitations due to key aspects of their life cycle–amphibians, like fish, require water for reproduction. The eggs of these animals must be laid in water because they do not have any sort of protective outer coating to protect the egg from drying out. Additionally, early life stages after hatching (for example, a tadpole stage in frogs) require water until the organism develops enough to survive on land.
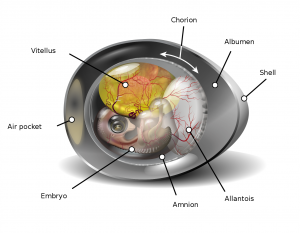
During the Carboniferous, a major innovation arose in tetrapod groups–the amniotic egg. An amniotic egg protects a developing embryo and its required nourishment (yolk sac) within several protective layers. These layers provide protection from environmental elements, and also allow for the transmission of gases needed for respiration and a place to store metabolic wastes. Whether the outermost shell layer is leathery or more hardened, this change to eggs allowed tetrapods to spread out into different inland environments, further away from permanent water sources. Amniotes, as a clade, are represented by reptiles (including dinosaurs and birds) and mammals.
This ability to exploit more terrestrial environments allowed amniote groups to diversify rapidly and persist for millions of years, with natural selection aided by the constant shift of tectonic plates and resulting climate patterns. During the Late Carboniferous, two distinct lineages of tetrapods arose–the sauropsids and synapsids. Within the sauropsid clade, an enormous diversity of body forms and life habits arose, particularly after the end-Permian extinction cleared out a variety of ecological niches and allowed reptiles to undergo an adaptive radiation. This lineage leads to reptilian groups like crocodilians, turtles, snakes, dinosaurs, and dinosaurs’ cousins, birds. The end-Cretaceous mass extinction wiped out a significant number of the reptilian groups, effectively wiping the slate clean for emerging mammalian groups to repopulate ecological niches on land, air, and sea.
The Late Carboniferous synapsids began with a reptilian-like, low-slung body plan, and a distinctive single opening in the skull behind the eye socket. Many of the larger early members of the synapsid group such as Dimetrodon and Cynognathus were greatly affected by the end-Permian and end-Triassic mass extinctions. Eventually, changes within this clade led to the various groups of mammals we see today, including monotremes (egg-laying mammals, like platypus), marsupials (pouched mammals, like koalas and kangaroos) and placental mammals (most mammals, including such diverse groups as cows, whales, cats, and humans).
While vertebrate groups on land were evolving throughout the Mesozoic, so were vascular plants. The ability to reproduce via seeds had developed during the Devonian among gymnosperms (seed ferns, and eventually conifers, cycads, and ginkgos), but it was millions of years later, during the Cretaceous, when flowering plants (angiosperms) evolved. Angiosperms are the most common plants on Earth today and includes grasses, common trees like oaks and maples, herbs, and all of our domesticated crops, such as corn, rice, and soybeans.
Gymnosperms primarily reproduce via wind-blown pollen released from cones. Flowers on angiosperms are reproductive structures that allow for pollen distribution by organisms (e.g., insects like bees) and are often colorful, showy features that attract organisms for gamete dispersal. Gymnosperm seeds are literally “naked seeds”–the seed is not encased in an ovary. However, on angiosperms, (“vessel seeds”) the plant’s seeds are encased in ovaries we know as fruit. So the more fruit that is produced and eaten, the more seeds are dispersed via animal dung, continuing the plant population, and potentially altering the plant’s geographic distribution. These tightly woven relationships between plants, pollinators, and dispersers represent some of the most elegant examples of coevolution throughout geologic time.
The interactions of living organisms clearly play a key role in how Earth has changed during its 4.6 billion year history. Likewise, interactions with the atmosphere, hydrosphere, and geosphere have shaped and pruned the tree of life. As humans, we are part of the unique, weird, beautiful, fascinating,opportunistic, innovative, and simply wonderful story of life on Earth.
Further reading and resources
- Buatois, L.A. and Buatois, L.A. 2016. “Ediacaran Ecosystems and the Dawn of Animals,” in Mángano, M.G. and Buatois, L.A. (eds.) The Trace-Fossil Record of Major Evolutionary Events. Topics in Geobiology, volume 39. DOI 10.1007/978-94-017-9600-2_2
- Wei-Hass, M. 2018. “World’s Oldest Fossils May Just Be Pretty Rocks,” National Geographic.
- Knoll, A. H. 2015. Life on a Young Planet: The First Three Billion Years of Evolution on Earth. Princeton University Press.
- The Shape of Life, a website dedicated to “The Story of the Animal Kingdom.”
- Shaw, S.R. 2014. Planet of the Bugs: Evolution and the Rise of Insects. University of Chicago Press.
- Shubin, N. 2008. Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body. Pantheon Press.
- Annie McEwen, Bethel Habte, Latif Nasser, Matt Kielty, Simon Adler and Tracie Hunte (2017). “Bigger Little Questions,” Radiolab podcast/episode, WNYC.
Citations
- Abramov, O. and Mojzsis, S.J. 2009. Microbial habitability of the Hadean Earth during the Late Heavy Bombardment. Nature 459 (7245): 419-22. doi: 10.1038/nature08015
- Alleon, J., S. Bernard, S., Le Guillou, C., Beyssac, O., Sugitan, K., Robert, F . 2018. Chemical nature of the 3.4 Ga Strelley Pool microfossilsGeochemical Perspective Letters 7:37-42. doi: 10.7185/geochemlet.1817
- Allwood, A.C., Rosing, M.T., Flannery, D.T., Hurowitz, J.A., and Heirwegh, C.M. 2018. Reassessing evidence of life in 3,700-million-year-old rocks of Greenland. Nature 563: 241–244. doi: 10.1038/s41586-018-0610-4
- Bekker, A., Sokur, T., Shumlyanskyy, L., Junium, C.L. Podkovyrov, V., Kuznetsov, A., Love, G.D., Pehr, K. 2018. Ediacara biota flourished in oligotrophic and bacterially dominated marine environments across Baltica. Nature Communications. 9(1): 1807. doi: 10.1038/s41467-018-04195-8
- Bobrovskiy, I., Hope, J.M., Ivantsov, A.,; Nettersheim, B.J., Hallmann, C., Brocks, J.J. 2018. Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals. Science 361 (6408): 1246–1249. doi:10.1126/science.aat7228
- Boraas, M.E., Seale, D.B. and Boxhorn, J.E. 1998. Phagotrophy by a flagellate selects for colonial prey: a possible origin of multicellularity. Evolutionary Ecology 12: 153–164.
- Herron, M.D., Borin, J.M., Boswell, J.C., Walker, J., Chen, I.K., Knox, C.A., Boyd, M., Rosenzweig, F., and Ratcliff, W.C. 2019. De novo origins of multicellularity in response to predation. Scientific Reports 9: 2328.
- Dodd, M.S., Papineau, D., Grenne, T., Slack, J.F., Rittner, M., Pirajno, F., O’Neil, J. and Little, C.T.S. 2017. Evidence for early life in Earth’s oldest hydrothermal vent precipitates. Nature 543: 60-64.
- Jokela, J. ,Dybdahl, M.F., and Lively, C.M. 2009. The Maintenance of Sex, Clonal Dynamics, and Host-Parasite Coevolution in a Mixed Population of Sexual and Asexual Snails. The American Naturalist 174 (s1): S43. doi: 10.1086/599080
- Marchi, S., Bottke, W.F., Elkins-Tanton, L.T., Bierhaus, M., Wuennemann, K., Morbidelli, A., Kring, D.A. 2014. Widespread mixing and burial of Earth’s Hadean crust by asteroid impacts. Nature 511(7511): 578-582. doi:10.1038/nature13539
- Marshall, C., Emry, J., and Olcott Marshall, A. 2011. Haematite pseudomicrofossils present in the 3.5-billion-year-old Apex Chert. Nature Geoscience 4: 240–243. doi: 10.1038/ngeo1084
- Nutman, A., Bennett, V., Friend, C., Van Kranendonk, M.J., Chivas, A.R. 2016. Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures. Nature 537: 535–538. doi.org/10.1038/nature19355
- Schopf, J. W. 1993. Microfossils of the early Archean Apex Chert: New evidence of the antiquity of life. Science 260, 640–646 (1993).
- Wacey, D., Kilburn, M.R., Saunder, M., Cliff, J. and Brasier, M.D. 2011. Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia. Nature Geoscience 4: 698-702.