In the beginning…
How and when does the story of Earth begin? A logical place to start is with the formation of the planet, but as you’ll soon see, the formation of the planet is part of a larger story, and that story implies some backstory before the story, too. The purpose of this case study is to present our best scientific understanding of the formation of our solar system from a presolar nebula, and to put that nebula in context too.
Nebular theory
The prevailing scientific explanation for the origin of the Earth does a good job of not only explaining the Earth’s formation, but the Sun and all the other planets too. Really, it’s not “the Earth’s origin story” alone so much as it is the origin story of the whole solar system. Not only that, but our Sun is but one star among a hundred million in our galaxy, and our galaxy is one of perhaps a hundred million in the universe. So the lessons we learn by studying our own solar system can likely be applied more generally to the formation of other solar systems elsewhere, including those long ago, in galaxies far, far away. The vice versa is also true: Our understanding of our own solar system’s origin story is being refined as we learn more about exoplanets, some of which defy what we see in our own system; “hot Jupiters” and “super-Earths,” for instance, are features we see in other star systems but not our own.
When we use powerful telescopes to stare out into the galaxy, we observe plenty of other stars, but we observe other things too, including fuzzy looking features called nebulae. A nebula is a big cloud of gas and dust in space. It’s not as bright as a star because it’s not undergoing thermonuclear fusion, with the tremendous release of energy that accompanies that process. An example of a nebula that you are likely to be able to see is in the constellation Orion. Orion’s “belt,” three stars in a row, is a readily identifiable feature in the northern hemisphere’s night sky in winter. A smaller trio of light spots “dangle” from the belt; this is Orion’s sword scabbard. A cheap pair of binoculars will let you examine these objects for yourself; you will discover that the middle point of light in this smaller trio is not a star. It is a nebula called Messier 42.
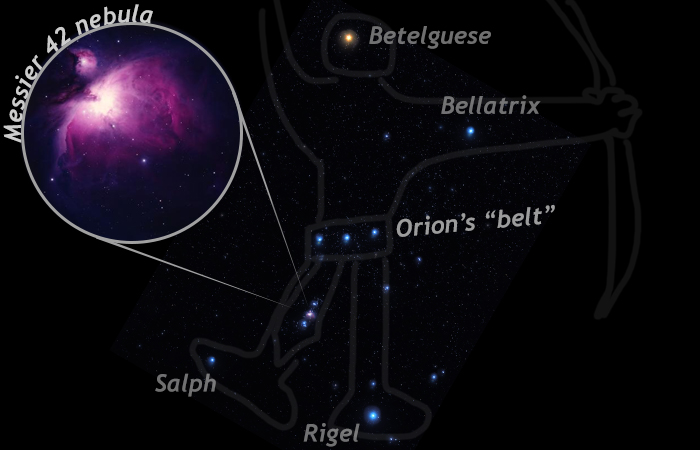
Nebulae like Messier 42 are common features of the galaxy, but not as common as stars. Nebulae appear to be short-lived features, as matter is often attracted to other matter. All that stuff distributed in that tremendous volume of space is not as stable as it would be if it were all to be drawn together into a few big clumps. Particles pull together with their neighboring particles under the influence of various forces, including “static cling” or electrostatic attraction. This is the same force that makes tiny dust motes clump up into dust bunnies under your couch!

Now, electrostatic force is quite strong for pulling together small particles over small distances, but if you want to make big things like planets and stars out from a nebula, you’re going to need gravity to take over at some point. Gravity is a rather weak force. After all: every time you take a step, you’ve overcoming the gravitational pull of the entire Earth. But gravity can work very efficiently over distance, if the masses involved are large enough. So static cling was the initial organizer, until the “space dust bunnies” got large enough, then gravity was able to take over, attracting mass to mass. The net result is that the gajillions of tiny pieces of the nebula were drawn together, swirling into a denser and denser amalgamation. The nebula began to spin, flattening out from top to bottom, and flattening out into a spinning disk, something between a Frisbee and a fried egg in shape:

Once a star forms in the center, astronomers call the ring of debris around it a protoplanetary disk. Two important processes that helped organize the protoplanetary disk further were condensation and accretion.

Condensation is the process where gaseous matter sticks together to make liquid or solid matter. We have evidence of condensation in the form of small spherical objects with internal layering, kind of like “space hailstones.” These are chondrules, and they represent the earliest objects formed in our solar system. (Occasionally, we are lucky enough to find chondrules that have survived until the present day, entombed inside certain meteorites of the variety called chondrites.)
Chondrules glommed onto other chondrules, and stuck themselves together into primordial “rocks,” building up larger and larger objects. Eventually, these objects got to be big enough to pull their mass into an round shape, and we would be justified to dub them “planetesimals.” Planetesimals gobbled up nearby asteroids, and smashed into other planetesimals, merging and growing through time through the process of accretion. The kinetic force of these collisions heated the rocky and metallic material of the planetesimals, and their temperature also went up as radioactive decay heated them from within. Once warm, denser material could sink to their middles, and lighter-weight elements and compounds rose up to their surface. So not only were they maturing into spheroidal shapes, but they were also differentiating internally, separating into layers organized by density.

Meteorites that show metallic compositions represent “core” material from these planetesimals; core material that we would never get to glimpse had not their surrounding rocky material been blasted off. Iron meteorites such as the Canyon Diablo meteorite below (responsible for Arizona’s celebrated Meteor Crater) therefore are evidence of differentiation of planetesimals into layered bodies, followed by disaggregation: a polite way of saying they were later violently ripped apart by energetic collisions.
If you were to somehow weigh the nebula before condensation and accretion, and again 4.6 billion years later, we’d find the mass to be the same. Rather than being dispersed in a diffuse cloud of uncountable atoms, the condensation and accretion of the nebula resulted in exactly the same amount of stuff, but organized into a smaller and smaller number of bigger and bigger objects. The biggest of these was the Sun, comprising about 99.86% of all the mass in the solar system. Four-fifths of the remaining 0.14% makes up the planet Jupiter. Saturn, Neptune, and Uranus are huge gas giants as well. The inner rocky planets (including Earth) make up a tiny, tiny fraction of the total mass of the whole solar system – but of course, just because they are relatively small, that doesn’t mean they are unimportant!
The process of accretion continues into the present day, though at a slower pace than the earliest days of the solar system. One place you can observe this is in the asteroid belt, where there are certain asteroids that are basically nothing more than a big 3D pile of space rocks, held together under their own gravity. Consider the asteroid called Itokawa 25143, for instance:
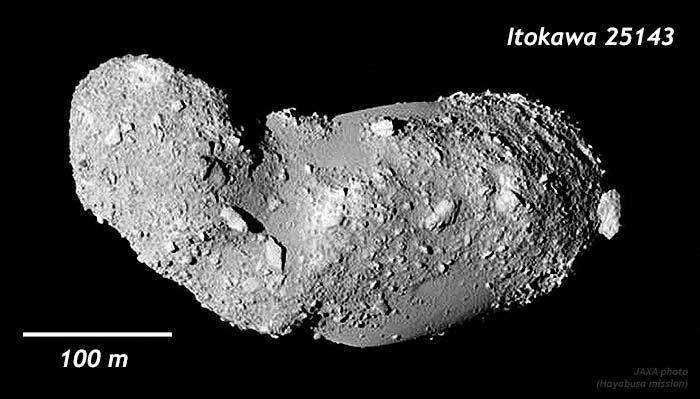
Only about half a kilometer long, and only a few hundred meters wide, Itokawa doesn’t even have enough gravity to pull itself into a sphere. If you were to land on the surface of Itokawa and kick a soccer-ball-sized boulder, it would readily fly off into space, as the force of your kick would be much higher than the force of gravity causing it to stay put.
Another example of accretion continuing to this day is meteorite impacts. Every time a chunk of rock in space intersects the Earth, its mass is added to that of the planet. In that instant, the solar system gets a little bit cleaner (fewer leftover bits rattling around) and the planet gets a little more massive. A spectacular example of this occurred in 1994 with Comet Shoemaker-Levy 9, a comet which had only been discovered the previous year. Jupiter’s immense gravity broke the comet into chunks, and then swallowed them up one after another. Astronomers on Earth watched with fascination as the comet chunks, some more than a kilometer across, slammed into Jupiter’s atmosphere at 60 km/second (~134,000 mph), creating a 23,700°C fireball and enormous impact scars that were as large as the entire Earth. These scars lasted for months.
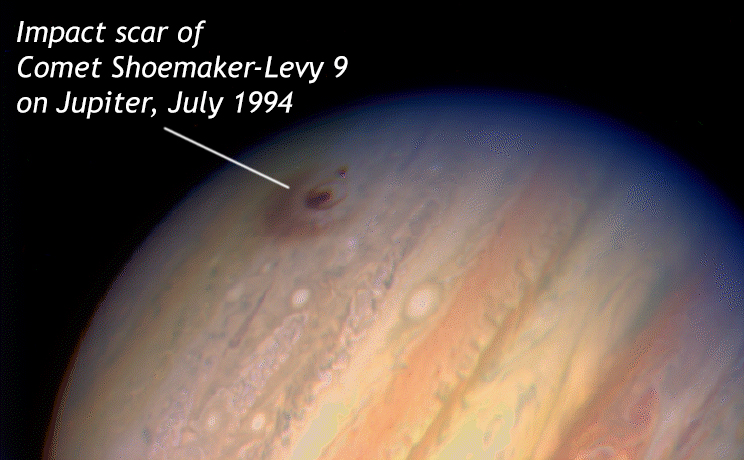
This incredibly dramatic event perhaps raises the hair on our necks, seeing the violence and power of cosmic collisions. It’s a reminder that Earthlings are not safe from accretionary impacts even today – as the dinosaurs found out. For the purposes of our current discussion, though, bear in mind that the collision was really a merger between the masses of Comet Shoemaker-Levy 9 and the planet Jupiter, and after the dust settled, the solar system had one fewer object left off by itself, and Jupiter gained a bit more mass. This is the overall trend of the accretion of our solar system from the presolar nebula: under gravity’s influence, the available mass becomes more and more concentrated through time.
Did I get it?
A star is born
Because the Sun is so massive, it is able to achieve tremendous pressures in its interior. These pressures are so high, they can actually force two atoms into the same space, overcoming their immense repulsion for one another, and causing their two nuclei to merge. As two atoms combine to make one more massive atom, energy is released. This process is thermonuclear fusion. Once it begins, stars begin to give off light.
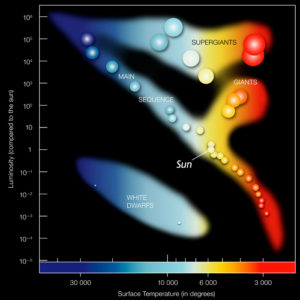
The ability of stars to make big atoms from small ones is key to understanding the history of our solar system and our planet. Planet Earth is made of a wide variety of chemical elements, both lightweight and heavy. All of these elements must have been present in the nebula, in order for them to be included in Earth’s “starting mixture.” Elements formed in the Sun today stay in the Sun, fusing low-weight atoms into heavier atoms. So all the elements on Earth today came from a pre-Sun star. We can go outside on a spring day and enjoy the Sun’s warmth, but the carbon that makes up the skin that basks in that warmth was forged in the heart of another star, a star that’s gone now, a star that blew up.
This exploding star was the source of the nebula where we began this case study: it’s the backstory that occurred before the opening scene. Our solar system is like a “haunted house,” where billions of years ago, there was a vibrant, healthy main-sequence star right here, in this part of the galaxy. Perhaps it had planets orbiting it. Perhaps some of those planets harbored life. We’ll never know: the explosion wiped the slate clean, and “reset” the solar system for the iteration in which we live. The ghostly remnants of this time before our own still linger, in the very stuff we’re made from. This long-dead star fused hydrogen to build the carbon in our bodies, the iron in our blood, the oxygen we breathe, and the silicon in the rocks of our planet.
This is an incredible realization to embrace: everything you know, everything you trust, everything you are, is stardust.
Did I get it?
Age of the solar system
So just when did all this happen? An estimate for the age of the solar system can be made using isotopes of the element lead (Pb). There are several isotopes of lead, but for the purposes of figuring out the age of the solar system, consider these four: 208Pb, 207Pb, 206Pb, and 204Pb.
208Pb, 207Pb, 206Pb are all radiogenic: that is to say, they stable “daughter” isotopes that are produced from the radioactive “parent” isotopes. Each is produced from a different parent, at a different rate:
| Parent isotope | Stable daughter | Half-life |
| 232Th | 208Pb | 14.0 b.y. |
| 238U | 206Pb | 4.5 b.y. |
| 235U | 207Pb | 0.70 b.y. |
204Pb is, as far as we know, non-radiogenic. It’s relevant to this discussion because it can serve as a ‘standard’ that can allow us to compare the other lead isotopes to one another. Just as if we wanted to compare the currencies of Namibia, Indonesia, and Chile, we might reference all three to the U.S. dollar. The dollar would serve as a standard of comparison, allowing us to better see the value of the Namibian currency relative to the Indonesian currency and the Chilean currency. That’s what 204Pb is doing for us here.
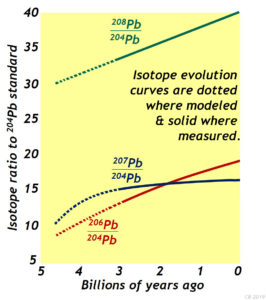
This is a plot showing the modeled evolution of our three radiogenic lead isotopes relative to 204Pb. It is constrained by terrestrial lead samples at the young end, and projected back in time in accordance with our measurements of how quickly these three isotopes of lead are produced by their radioactive parents. Of course, if we go back far enough in time, we run out of samples to evaluate. The Earth’s rock cycle has destroyed all its earliest rocks. They’ve been metamorphosed, or weathered, or melted – perhaps many times over! What would be really nice is to find some rocks from the early end of these curves – some samples that could verify these projections back in time are accurate.
Such samples do exist! But they are not from the Earth so much as “from the Earth’s starting materials.” If the nebular theory is correct, then a few leftover scraps of the planet’s starting materials are found in the solar system’s asteroids. Every now and again, bits of these space rocks fall to earth, and if they survive their passage through the atmosphere, we may be lucky enough to collect them, and analyze them. We call these space rocks “meteors” as they streak through the atmosphere, heating through friction and oxidizing as they fall. Those that make it all the way to Earth’s surface are known as “meteorites.” They can be often be distinguished by their scalloped fusion crust, as with this sample:
3D model by Marissa Dudek
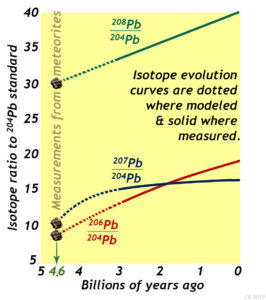
Meteorites come in several varieties, including rocky and metallic versions. It is very satisfying that when measurements of these meteorites’ lead isotopes are added to the plot above, they all fall exactly where our understanding of lead isotope production would have them: at the start of each of these model evolution curves. Each lead isotope system tells the same answer for the age of the Earth, acting like three independent witnesses corroborating one another’s testimony. And the answer they all give is 4.6 billion years ago (4.6 Ga). That’s what 208Pb says. That’s what 207Pb says. And that’s what 206Pb says. They all agree, and they agree with the predicted curves based on terrestrial (Earth rock) measurements. This agreement gives us great confidence in this number. The Earth, and meteorites (former asteroids), and the solar system of which they are all a part, began about 4.6 billion years ago…
Did I get it?
…But what came before that?
The implications of meteorites
In 1969, a meteorite fell through Earth’s atmosphere and broke up over Mexico. A great many pieces of this meteorite were recovered and made available for scientific analysis. It turned out to be a carbonaceous chondrite, the largest of its kind ever documented. It was named the Allende (“eye-YEN-day”) meteorite, for the tiny Chihuahuan village closest to the center of the area over which its fragments were scattered.
One of the materials making up Allende’s chondrules was the calcium feldspar called anorthite. Anorthite is an extraordinarily common mineral in Earth’s crust, but the Allende anorthite was different. For some reason, it has a large amount of magnesium in it. When geochemists determined what kind of magnesium this was, they were surprised to find that it was mostly 26Mg, an uncommon isotope. The abundances of 25Mg and 24Mg were found to be about the same level as Earth rocks, but 26Mg was elevated by about 1.3%. And after all, magnesium doesn’t even “belong” in a feldspar. The chemical formula of anorthite is CaAl2Si2O8 – there’s no “Mg” spot in there. Why was this odd 26Mg in this chondritic anorthite?
One way to make 26Mg is the break-down of radioactive 26Al. The problem with this idea is that there is no 26Al around today. It’s an example of an extinct isotope: an atom of aluminum so unstable that it falls apart extremely rapidly. The half-life is only 717,000 years. But because these chondrules condensed in the earliest days of the solar system, there may well have been plenty of 26Al around at that point for them to incorporate. And Al, of course, is a key part of anorthite’s CaAl2Si2O8 crystal structure.
So the idea is that weird extra 26Mg in the chondrule’s anorthite could be explained by suggesting it wasn’t always 26Mg: Instead, it started off as 26Al ,and it belonged in that crystal’s structure. However, over a short amount of time, it all fell apart, and that left the 26Mg behind to mark where it had once been. If this interpretation is true, it has shocking implications for the story of our solar system.
To understand why, we first need to ask, what came before the nebula? What was the ‘pre-nebula’ situation? Where did the nebula come from, anyhow?
It turns out that nebulae are generated when old stars of a certain size explode.
Light traveling outward after a nova-like explosion from the star V838 Monocerotis.
These explosions are called supernovae (the plural of supernova). The “nova” part of the name comes from the fact that they are very bright in the night sky – an indication of how energetic the explosion is. They look like “new” stars to the casual observer. Supernovae occur when a star has exhausted its supply of lightweight fuel, and it runs out of small atoms that can be fused together under normal conditions. The outward-directed force ceases, and gravitationally-driven inward-directed forces suddenly dominate, collapsing the star in upon itself. This jacks up the pressures to unbelievably high levels, and is responsible for the nuclear fusion of big atoms – every atom heavier than iron is made instantaneously in the fires of the supernova.
That suite of freshly-minted atoms included a bunch of unstable isotopes, including 26Al.
And here’s the kicker: If the 26Al was made in a supernova, started decaying immediately, and yet enough was around that a significant portion of it could be woven into the Allende chondrules’ anorthite, that implies a very short amount of time between the obliteration of our Sun’s predecessor, and the first moments of our own. Specifically, the 717,000 year half-life of 26Al suggests that this “transition between solar systems” played out in less than 5 million years, conceivably in only 2 million years.
That is very, very quickly.
Did I get it?
Summary
In summary, the planet Earth is part of a solar system centered on the Sun. This solar system, with its star, its classical planets, its dwarf planets, and its “leftover” comets and asteroids, formed from a nebula full of elements in the form of gas and dust. Over time, these many very small pieces stuck together to make bigger concentrations of mass, eventually culminating in a star and a bunch of planets that orbit it. Asteroids (and asteroids that fall to Earth, called meteorites), are leftovers from this process. The starting nebula itself formed from the destruction of a previous star that had exploded in a supernova. The transition from the pre-Sun star to our solar system took place shockingly rapidly.
Further reading
Marcia Bjornerud’s book Reading the Rocks. Basic Books, 2005: 226 pages.
Jennifer A. Johnson (2019), “Populating the periodic table: Nucleosynthesis of the elements,” Science. : 474-478.
Lee, T., D. A. Papanastassiou, and G. J. Wasserburg (1976), Demonstration of 26Mg excess in Allende and evidence for 26Al, Geophysical Research Letters, 3(1), 41-44.
______________